Organic compounds
A technology for compounds and compositions, applied in organic chemistry, isotope introduction into organic compounds, steroids, etc., can solve problems such as long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
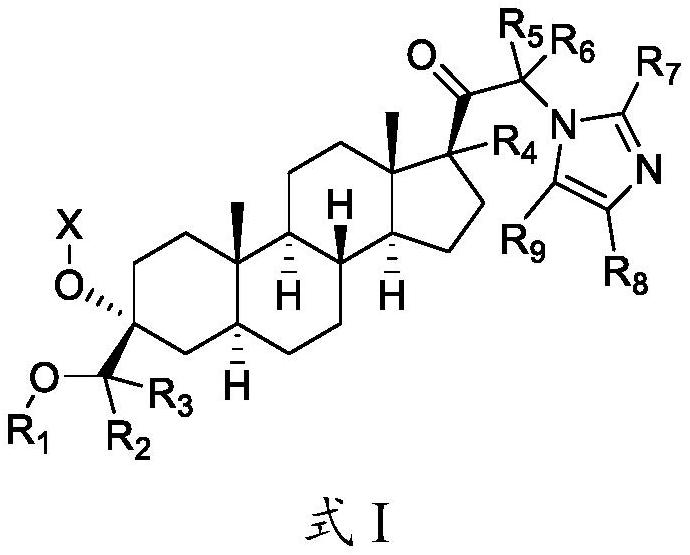
[0161] 3α-Hydroxy-3β-methoxymethyl-5α-pregnane-20-one can be synthesized from (3R)-spiro[oxirane-2α,5α-pregnane]-20-one and sodium methoxide such as Hogenkamp et al. People, "Synthesis and in Vitro Activity of 3β-Substituted-3α-hydroxypregnan-20-ones: Allosteric Modulators of the GABA A Prepared as described in Receptor"J Med.Chem.40:61-72 (1997). 21-substituted steroids can be prepared from the corresponding 21-bromosteroids using catalytic Br in MeOH of HBr 2Synthesized from 20-keto steroids. Other sources of useful synthetic methods include: Botella et al., J. Med Chem., 48:3500-3511 (2015); Botella et al., J. Med Chem., 60:7810-7819 (2017); Wong et al. , Steroids 71:77-82 (2006); Botella et al., WO 2016 / 061527; Hogenkamp, Derek L., WO 2000 / 66614; Goliber et al., US 2006 / 0074059; Chang et al., WO 2005 / 105822; Woodward, Richard M., WO 2006 / 131392; the contents of each of the aforementioned documents are hereby incorporated by reference in their entirety. In general, co...
Embodiment 1
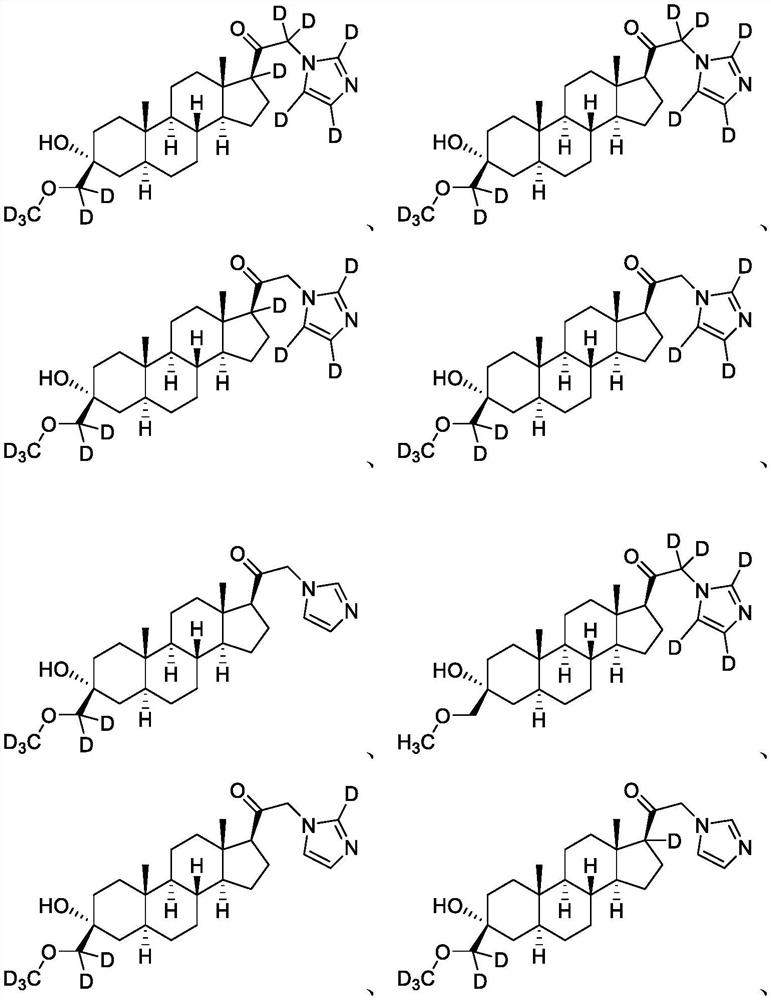
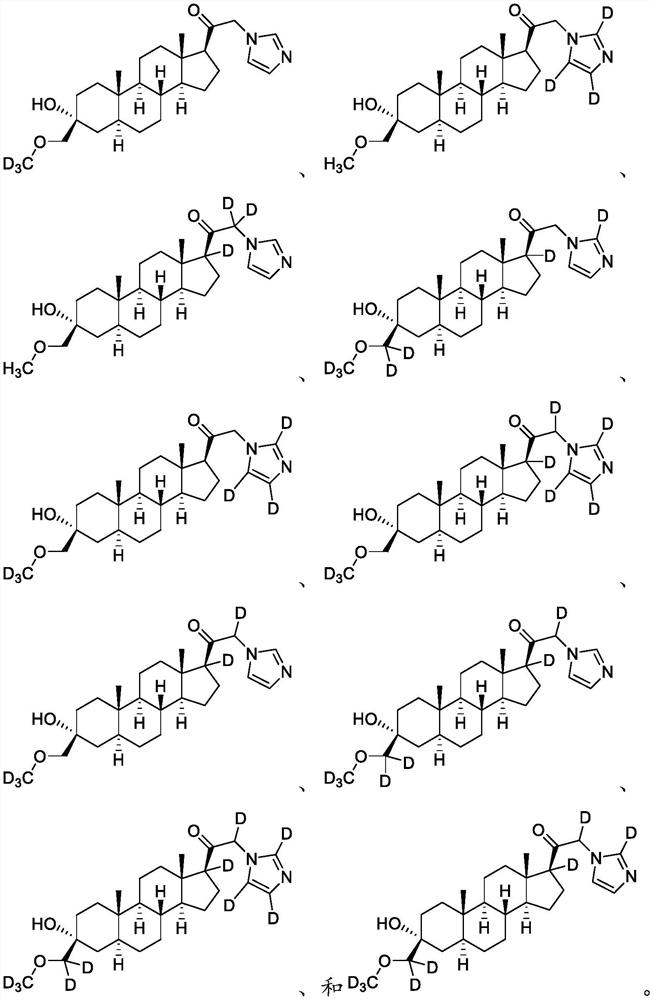
[0164] Example 1: Synthesis of 3α-hydroxyl-21-(1′-imidazolyl)-3β-methoxymethyl-5α-pregnane-20-one (compound of formula A)
[0165]
[0166] Step 1: 21-Bromo-3α-hydroxy-3β-methoxymethyl-5α-pregnane-20-one
[0167] To a stirred solution of 3α-hydroxy-3β-methoxymethyl-5α-pregna-20-one (30.0 g, 82.9 mmol) in 900 mL methanol at room temperature was added 3 drops of 48% HBr in water. A solution of bromine (13.9 g, 87.1 mmol) in 200 mL of methanol was then added dropwise over 2 hours, during which time the reaction was protected from light. After TLC (1% acetone / dichloromethane) indicated the absence of starting material and the formation of a less polar product, the reaction was concentrated to about 300 mL. Then add CH 2 Cl 2 (400 mL), the reactant was poured into a separatory funnel containing 200 mL of water. Separate the phases and wash the aqueous phase with CH 2 Cl 2 (3 x 100 mL) extraction. Combine the organic phases and wash with 200 mL saturated NaHCO 3 Washed wi...
Embodiment 2
[0170] Example 2: 1-((3R,5S,8R,9S,10S,13S,14S,17S)-3-hydroxyl-3-(methoxymethyl)-10,13-dimethylhexadecahydro- 1H-Cyclopentadien[a]phenanthrene-17-yl)-2-(1H-imidazol-1-yl-d3)ethan-1-one
[0171] imidazole-d 4 (0.193g, 2.72mmol, 3.0eq) To a 0°C solution in THF (2mL) was added lithium hydride (0.0237g, 2.8mmol, 3.1eq). The solution was stirred at 0 °C under Ar for 2 h. To the reaction mixture was slowly added 21-bromo-3α-hydroxy-3β-methoxymethyl-5α-pregnane-20-one (0.40 g , 0.906mmol, 1.0 equivalent). After stirring at 0 °C for 3 hours, the reaction mixture was quenched with methanol in an ice bath. The organic layer was separated and concentrated under reduced pressure. The residue was further purified by column chromatography to afford the pure title compound (0.184 g, 0.426 mmol) as a white powder in 50% isolated yield. 1 H NMR (500MHz, chloroform-d) δ4.86–4.60 (m, 2H), 3.41 (s, 3H), 3.20 (s, 2H), 2.60 (t, J=8.9Hz, 1H), 2.31–2.12 ( m,1H),2.06–1.90(m,1H),1.84–1.66(m,4H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com