Semen euphorbiae diterpene derivatives with MDR reversal activity and application of Semen euphorbiaediterpene derivatives
A technology of stephenia and derivatives, which is applied in the field of stephenia diterpene derivatives, and can solve the problems such as insignificant reversal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: Preparation and Determination of Compound 1 to 41
[0099] 1. Preparation of Compounds 1, 37, 40. In a 1% (m / V) NaOH / MeOH solution system, compounds 18, 38, 39 were respectively used as the raw material (Compound 18, in such a push), the normal temperature reaction was 24 h. After the reaction, it was extracted with ethyl acetate, concentrated, and the chromatography column was separated (eluting with EtOAc EtOAc). The core magnetic resonance data of Compound 1, 37, 40 is shown below.
[0100] Compound 1: HRMS-ESI-TOF: [M + Na] +373; MP 226-228 ° C; 1 H NMR (400 MHz, ACETONE) δ 7.29 (D, J = 11.6Hz, 1H, H-12), 5.07 (S, 1H, H-17α), 4.96 (D, J = 8.5 Hz, 1H, H-7 ), 4.81 (S, 1H, 17β), 4.68 (S, 1H, OH-15), 4.37 (S, 1H, H-3), 4.32 (S, 2H, OH-5, OH-7), 4.06 (S, 1H, OH-3), 3.07 (DD, J = 13.2, 8.8 Hz, 1H, H-1α), 2.21 (DD, J = 8.4, 3.3 Hz, 1H , H-4), 1.96-1.85 (M, 2H, H-2, H-8α), 1.69-1.61 (M, 4H, H-1β, H-20), 1.45 (DD, J = 11.6, 8.4 Hz , 1H, H-11), 1.31-1.25 (M, 2H, H-...
Embodiment 2
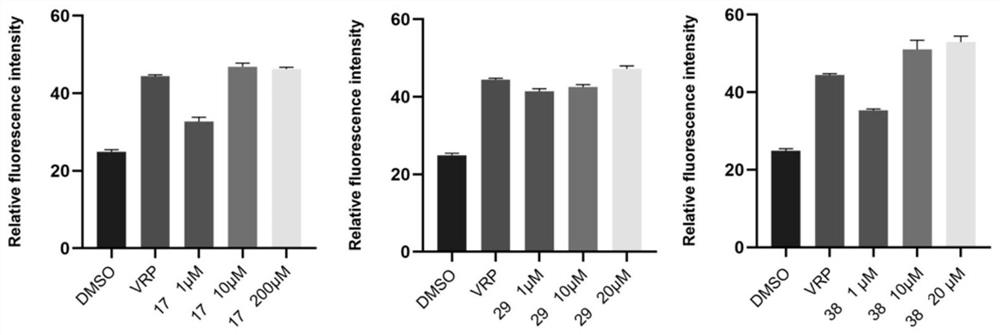
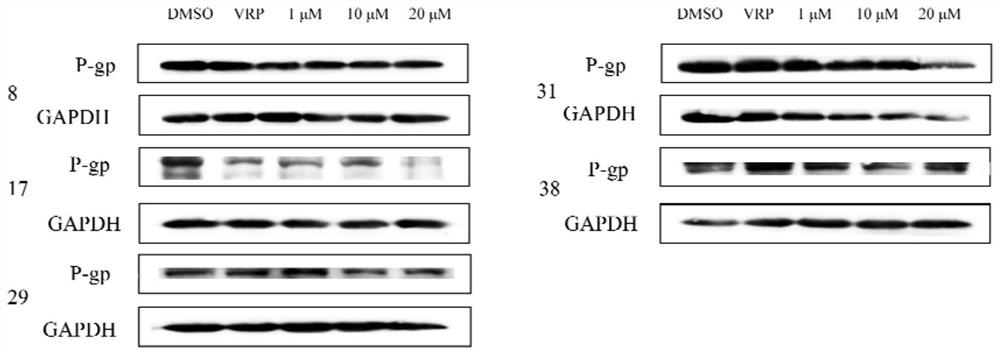
[0155] Example 2: Test of biological external activity of Compound 1 to 41
[0156]1, cytotoxic test of the hematoperpenoid derivatives. Specific operation: According to 1 × 110 / well, the MCF-7, MCF-7 / ADR cells are inoculated with 96-well plates, and 100 μl of medium medium containing 10% fetal bovine serum 15 h is added to the cell growth. To 70% of the coverage area, the final concentration gradient was added 1 μm, 5 μm, and the compound was dissolved with DMSO, and the drug volume was 1 μl. The final concentration gradient is 0.01 μm, 0.1 μm, 1 μm, 5 μm, and 10 μm, a multi-succytic star having a 10 μm as a positive control, and the compound DMSO is dissolved, and the administration volume is 1 μl. The DMSO is set for a negative control, and the administration volume is 1 μL. Each group sets 3 holes as parallel. After 48 hours of culture at 37 ° C, the original medium was removed, and the CCK-8 solution was diluted 10 times with a serum MEM medium, and 100 μl of dilution was...
Embodiment 3
[0167] Example 3: Orthopedic aggregation experiment
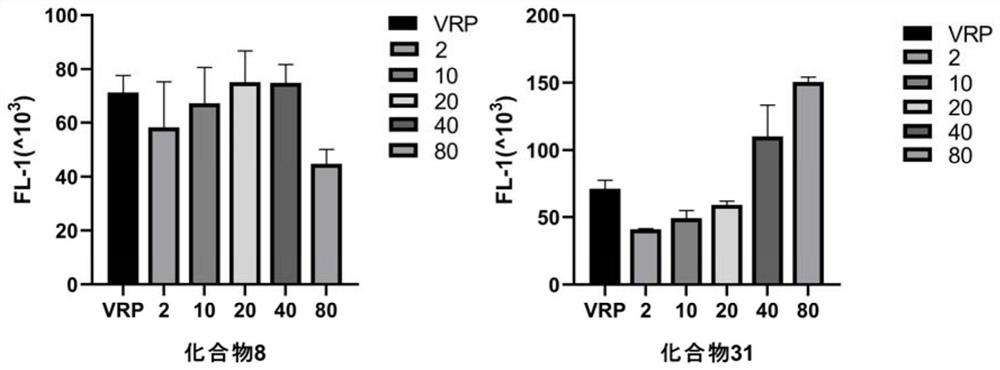
[0168] This example provides compounds 8, 31 for tonifying experimental tests in Rhodamin 123 (RH123). Specific operation: MCF-7 / ADR and MCF-7 cells in 2 × 10 6 The density of the aperture was inoculated into a 24-well plate, incubated for 16 h at 37 ° C, pretreatment of compound 8 and compound 31 (concentration of 2, 10 and 20 μM) with reversal resistance, and the final concentration of 20 μm. Villapimi as a positive control. The cells were then incubated with RH123 having a final concentration of 5 μm at 37 ° C to prevent light for 1 h. The cells were washed twice with PBS, resuspended in the PBS buffer, and analyzed by flow cytometry. Experimental results figure 1 Table 3 shows. figure 1 For the compound 8, the compound 31 accumulates the change in Rhodamine in MCF-7 / ADR cells, respectively.
[0169] Table 3 Compound 8, 31 inhibits P-GP-mediated rhodamine outbound experimental results display table
[0170]
[0171] Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com