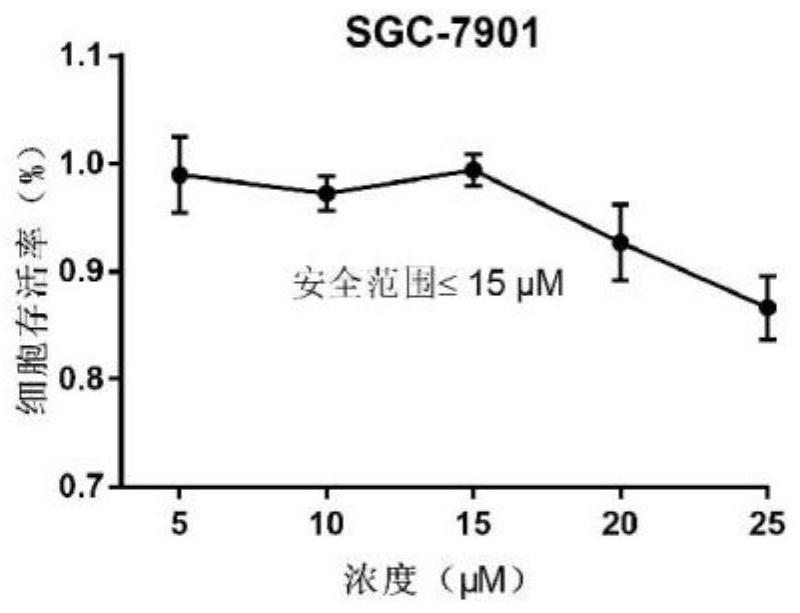
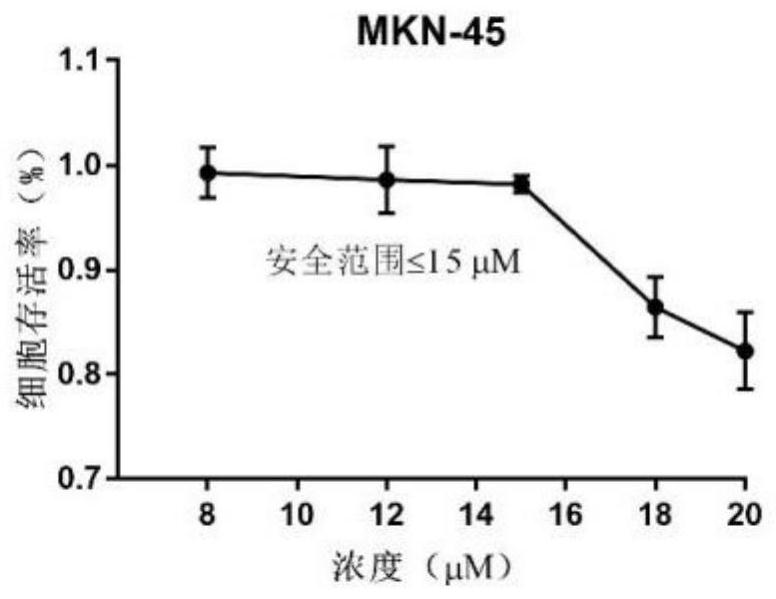
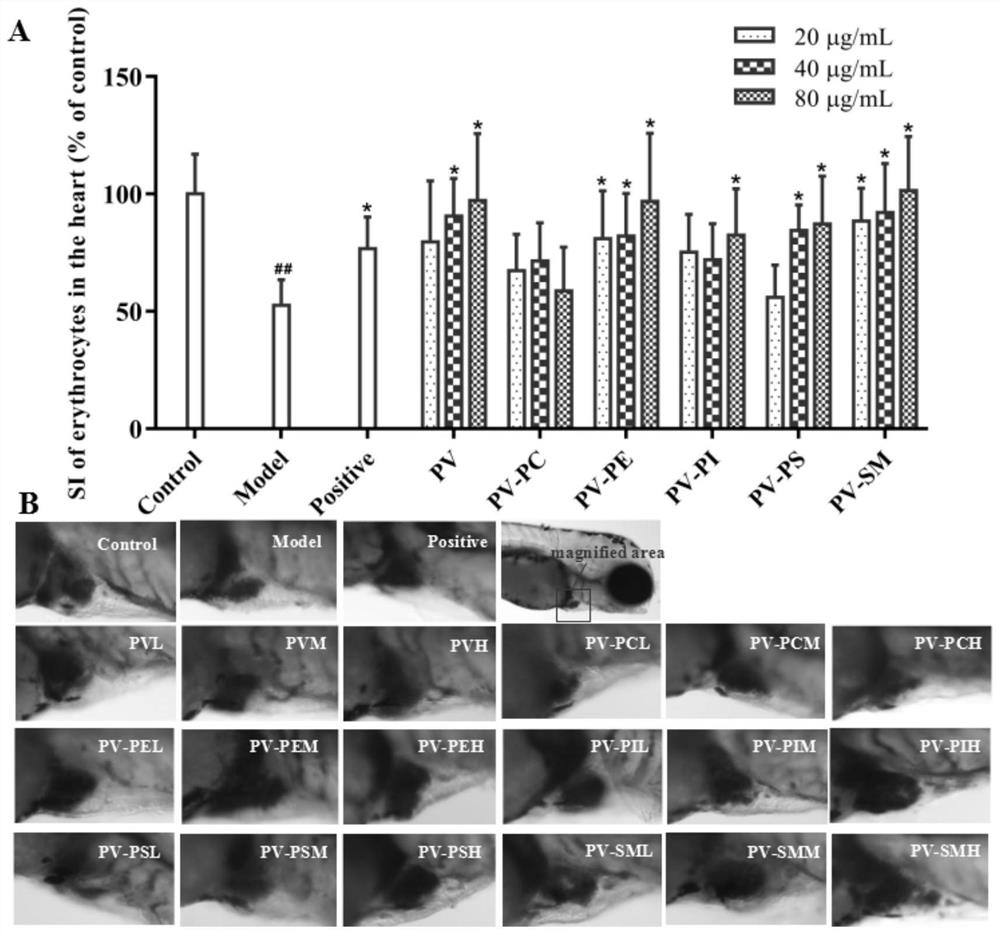
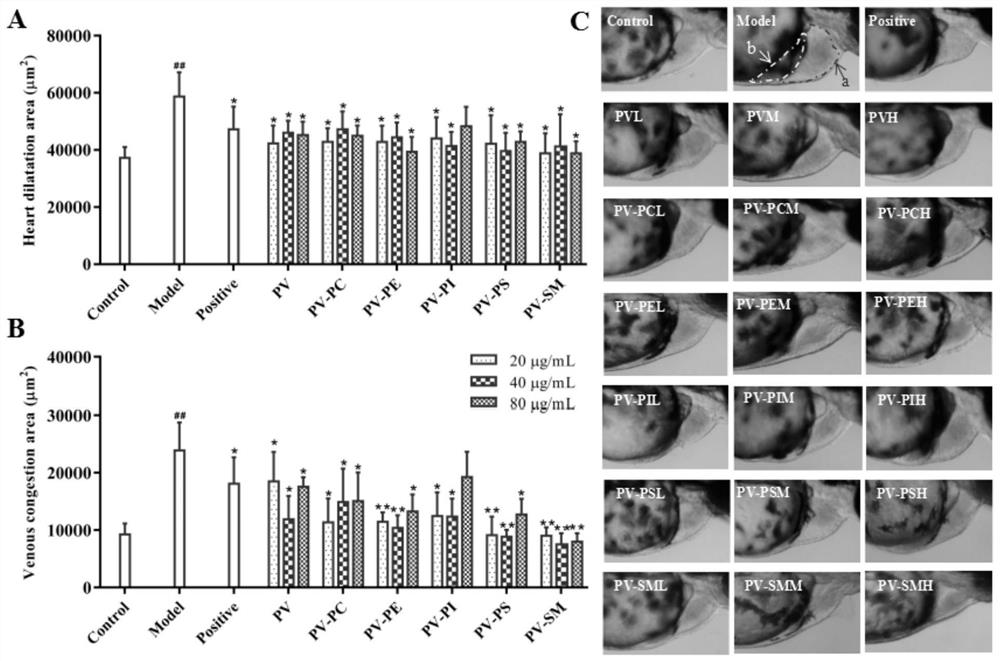
Patents
Literature
35 results about "Verapamilum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of bellidifolin in preparation of drugs for prevention and treatment of cardiacarrhythmia
InactiveCN102743374ASimplify operation stepsImprove extraction efficiencyOrganic active ingredientsCardiovascular disorderDrugPositive control
The present invention relates to application of bellidifolin and an extract thereof in preparations of drugs for prevention and treatment of cardiacarrhythmia. The present invention further discloses a drug composition, wherein the drug composition comprises bellidifolin adopted as an active ingredient or a pharmaceutically-acceptable carrier thereof, and an excipient or a diluent. Results of pharmacological experiments show that: the bellidifolin and the extract thereof have anti-cardiacarrhythmia effects, provide strong cardiacarrhythmia prevention activities compared with the control group, and provide the similar pharmacological properties compared with the positive control group of verapamil. In addition, the experimental study results of the present invention provide strong experimental supports for reasonable application of gentianella acuta traditional Chinese drug.
Owner:李旻辉
Verapamil hydrochloride delayed-release capsule and preparation method thereof
ActiveCN101422453ASmall inter-individual variabilityReduce or eliminate irritationNitrile/isonitrile active ingredientsCardiovascular disorderControlled releaseVerapamil Hydrochloride
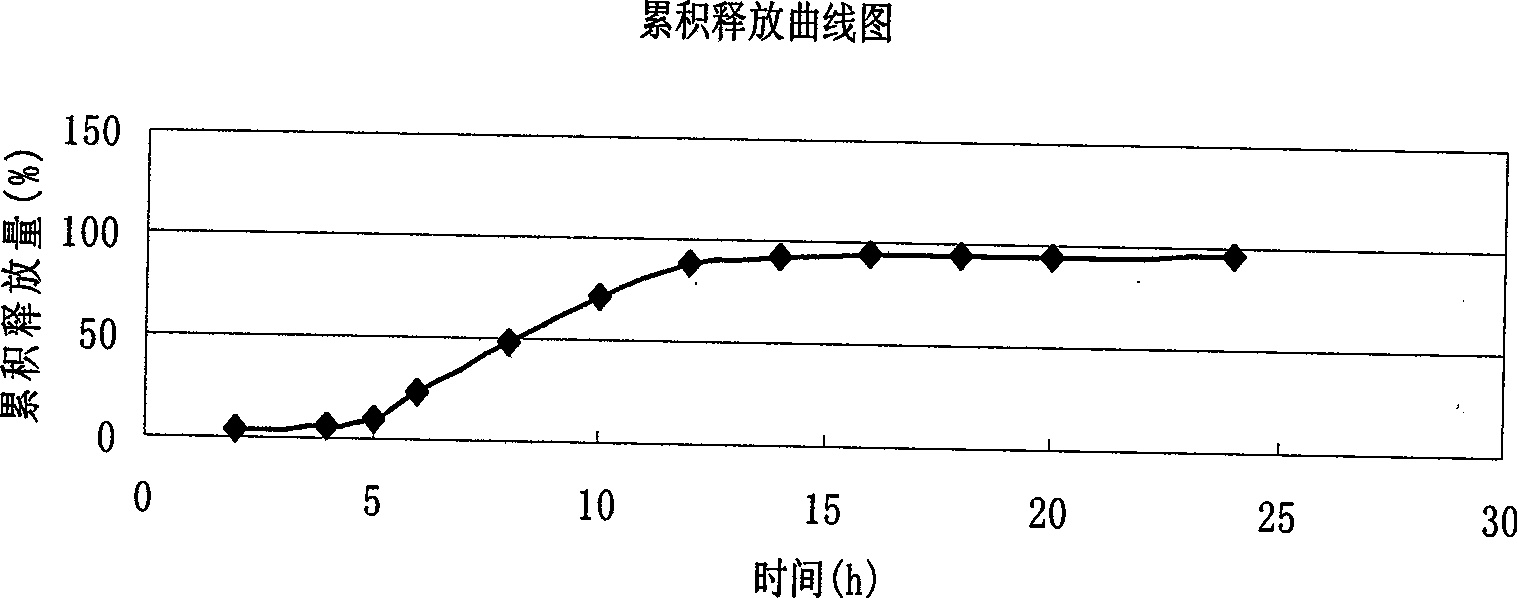
The invention provides a Verapamil Hydrochloride sustained release capsule; the weighting materials in the capsule are micropills; the micropill comprises a pill-containing core, an expansion layer and a controlled release layer from the interior to the exterior in turn; the weight of the pill-containing core accounts for 58 to 68 percent of the weight of the micropills; the expansion layer accounts for the 8 to 18 percent of the weight of the micropills; the controlled release layer accounts for 18 to 28 percent of the weight of the micropills; the Verapamil Hydrochloride sustained release capsule is a sustained release medicament-release system; the system can provide 4 to 6 hours of time lag and then slowly releases the medicament; more constant blood medicine concentration is maintained in a period of time; moreover, the preparation process of the capsule is simple and short; the production cost is lower; moreover, the Verapamil Hydrochloride sustained release capsule is beneficial to large industrial production.
Owner:LEPU PHARMACEUTICAL CO LTD
Moleplant seed diterpene derivatives and application thereof
ActiveCN113200950AExcellent reversal activityOrganic chemistryAntineoplastic agentsChemical compoundPerylene derivatives
The invention belongs to the field of organic chemistry, and particularly relates to moleplant seed diterpenoid derivatives with high MDR reversal activity and application thereof. The novel moleplant seed diterpene derivatives are extracted from euphorbia plant moleplant seeds, the compounds are subjected to structural modification, a series of compounds are designed and synthesized, and the reversal activity in MCF-7 / ADR cells is superior to that of verapamil, wherein the reversal multiple of the compound 29 is as high as 911.94.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Gene detection kit for guiding administration of antihypertensive drug verapamil
InactiveCN111172266AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationNucleotidePharmaceutical Substances
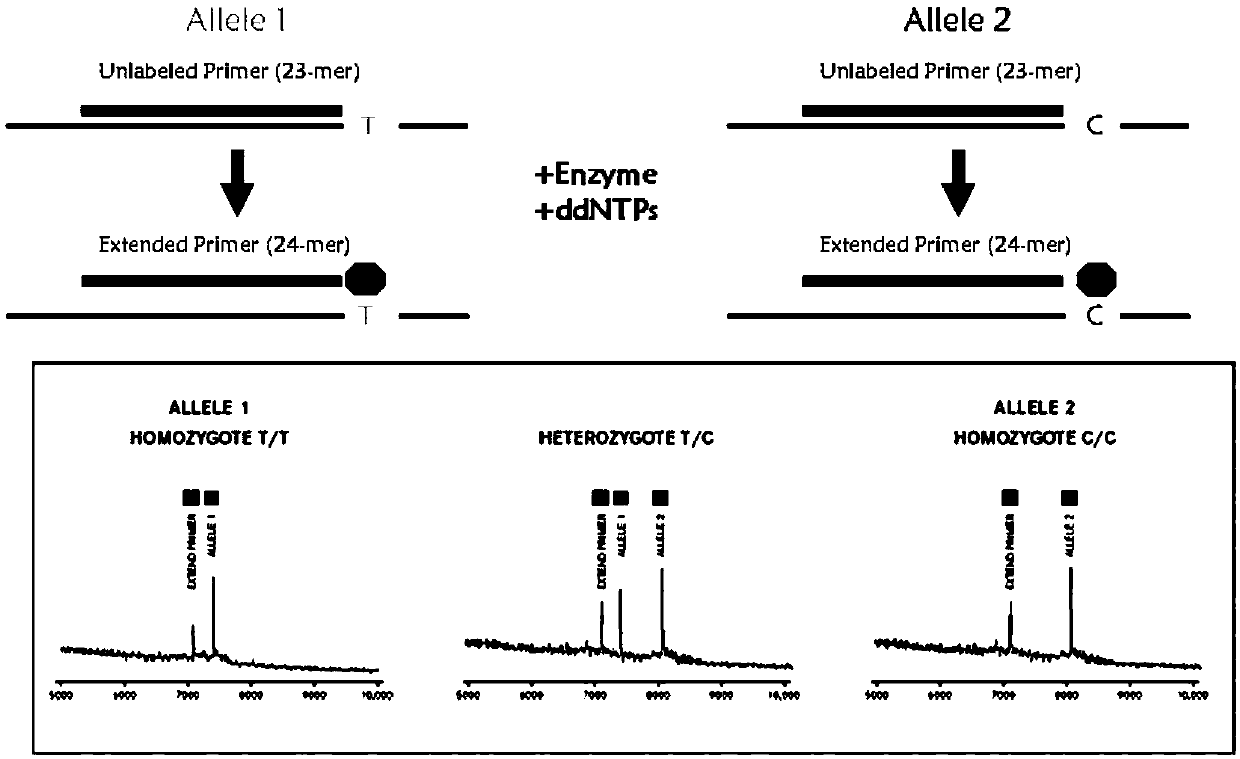
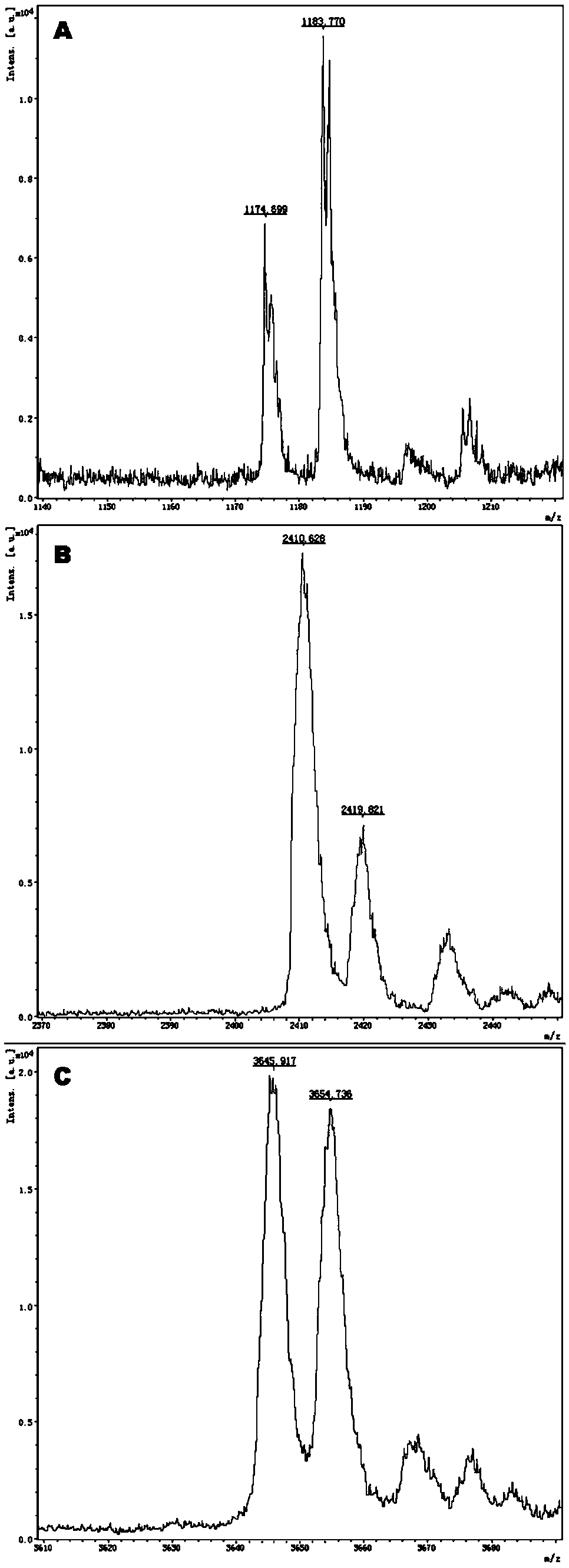
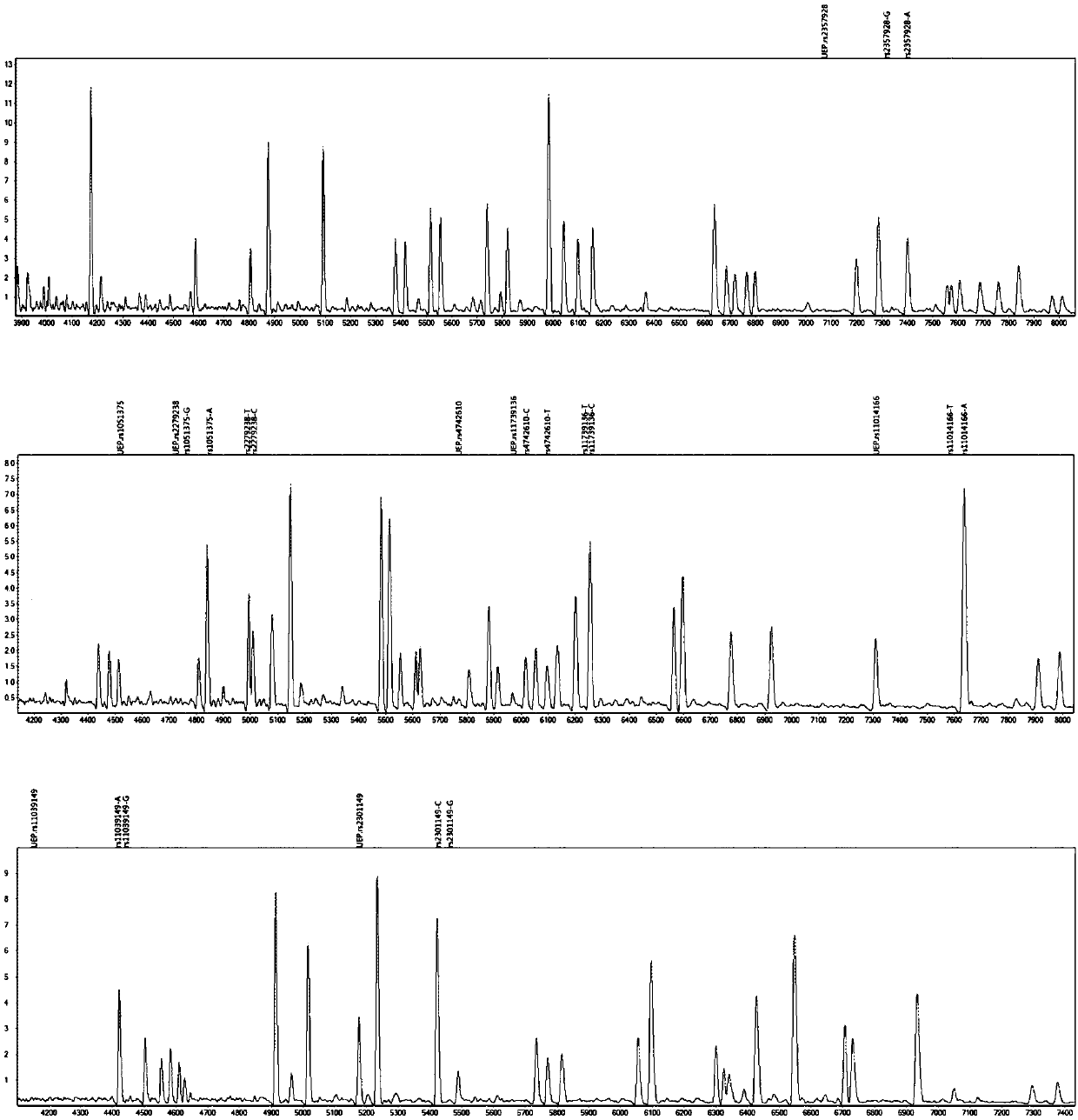
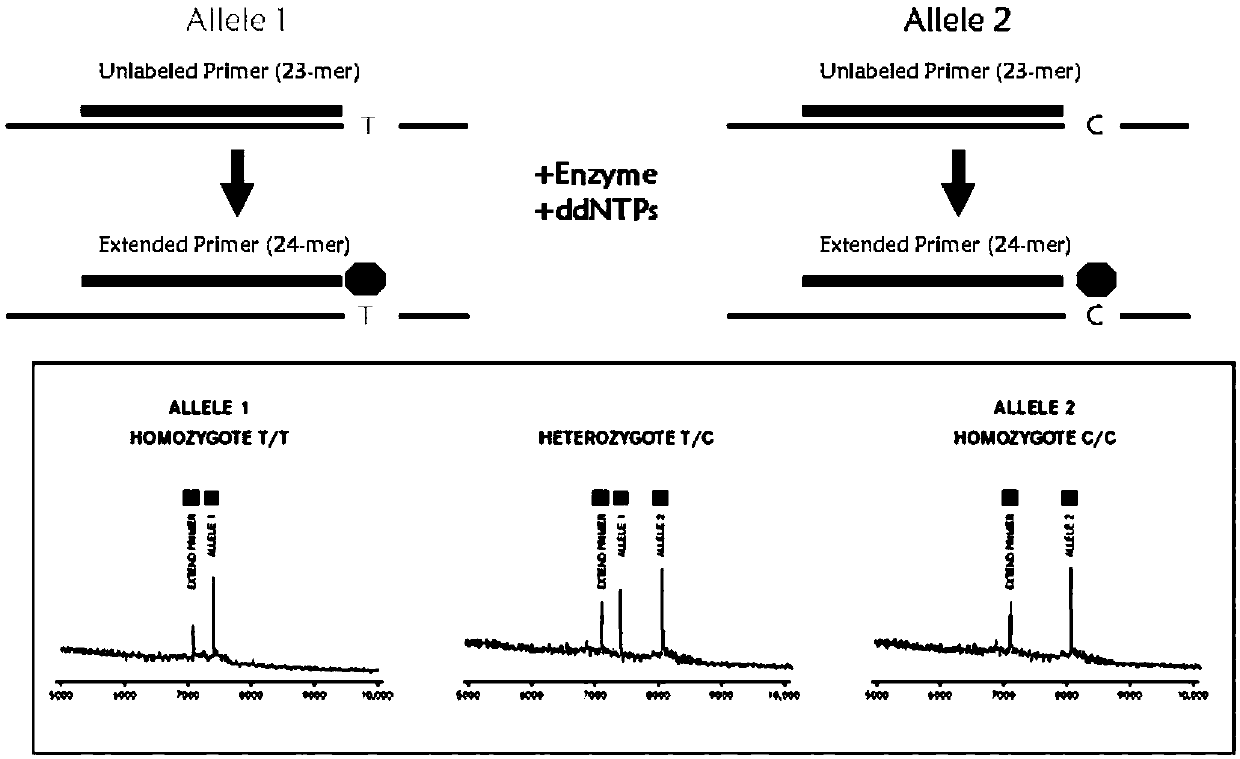
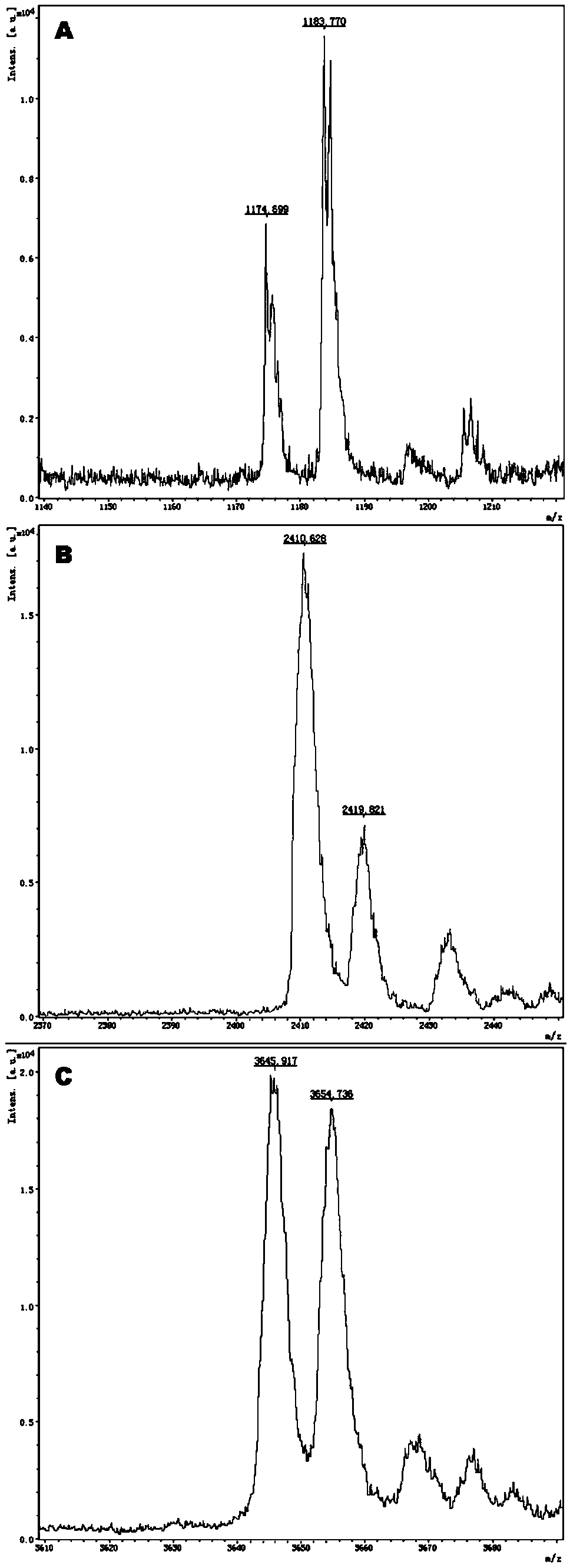
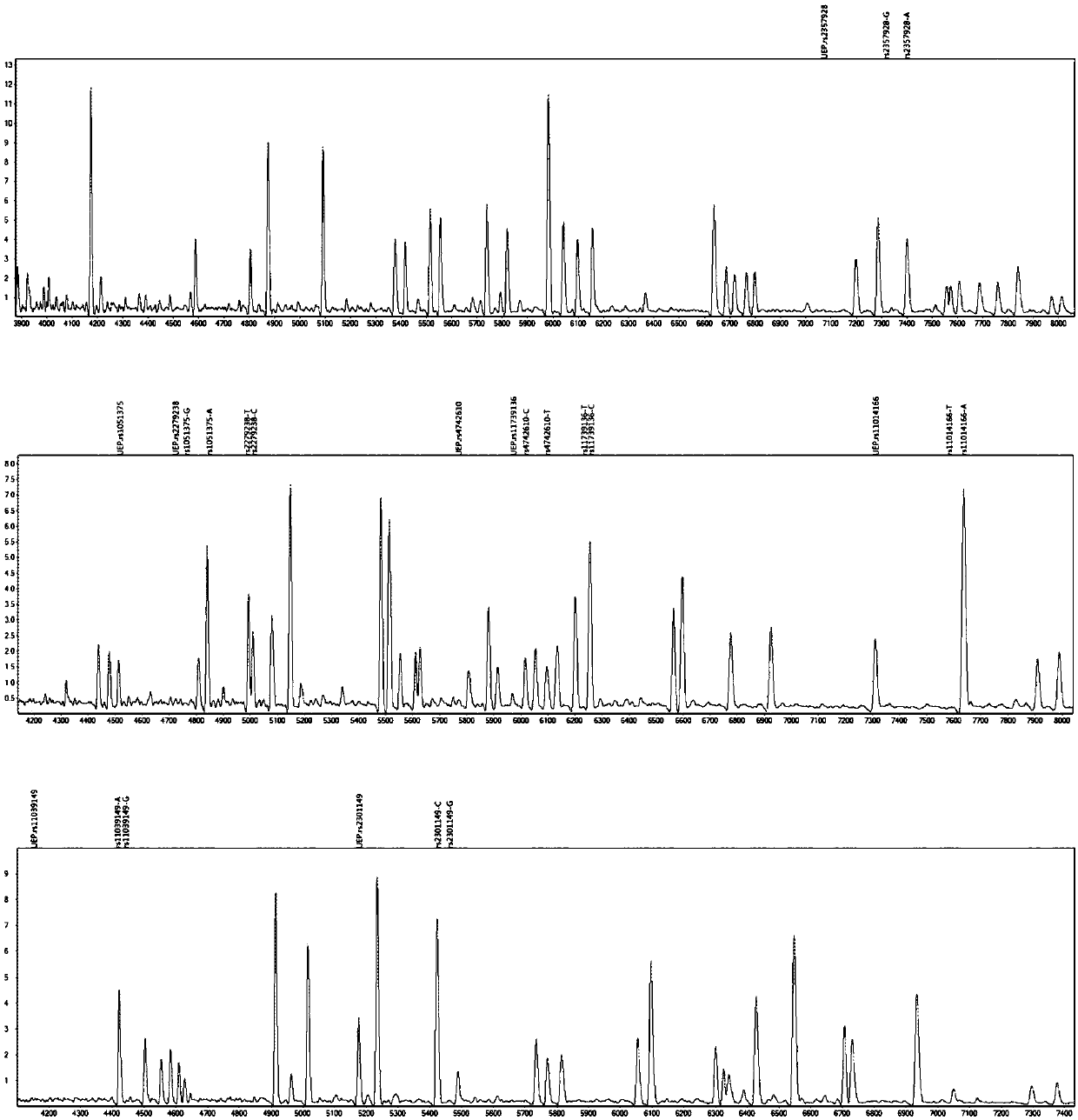
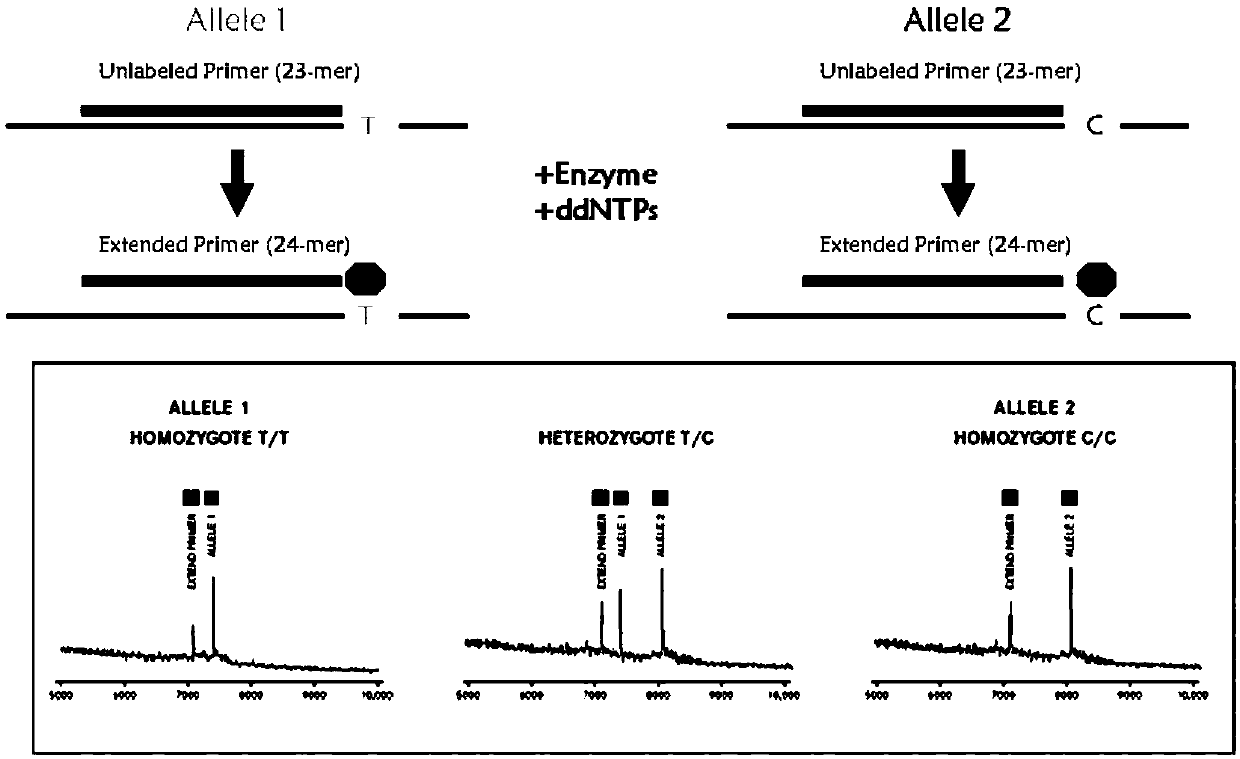
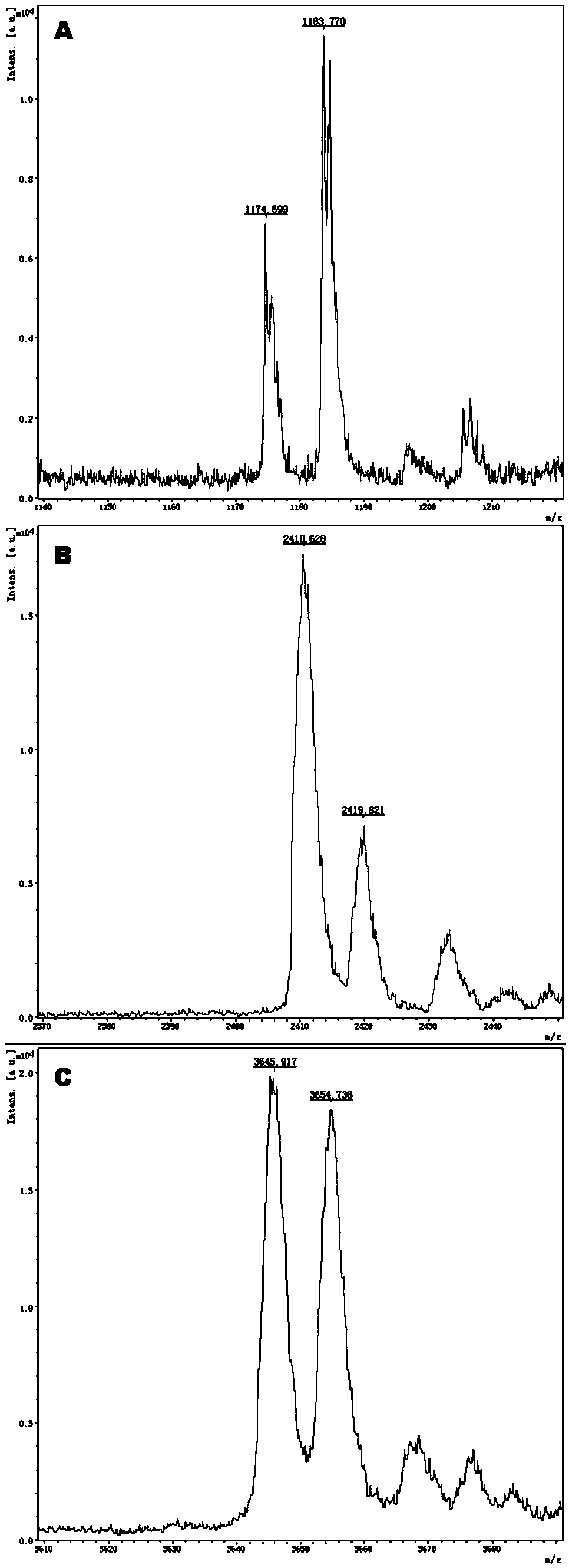
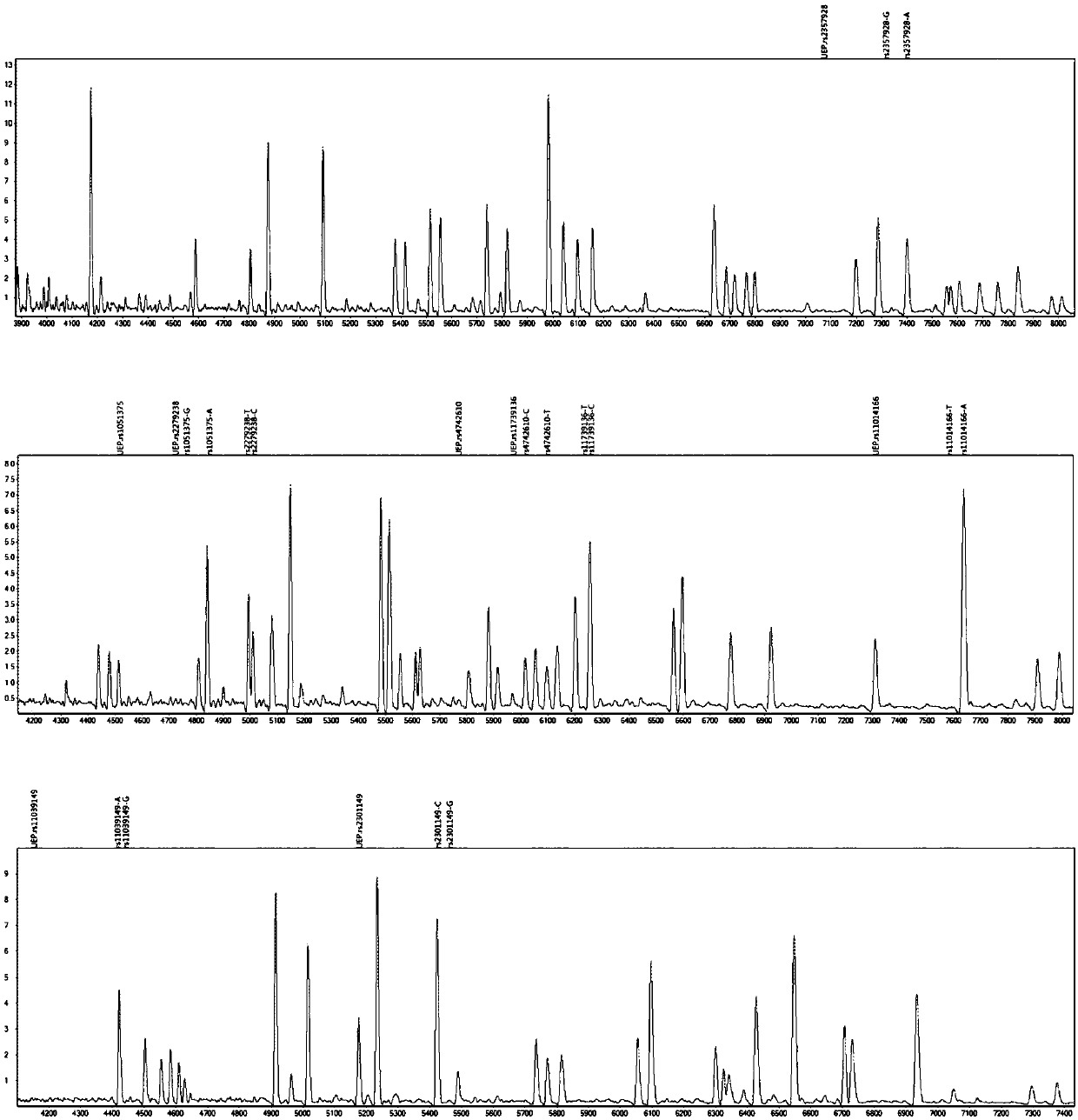
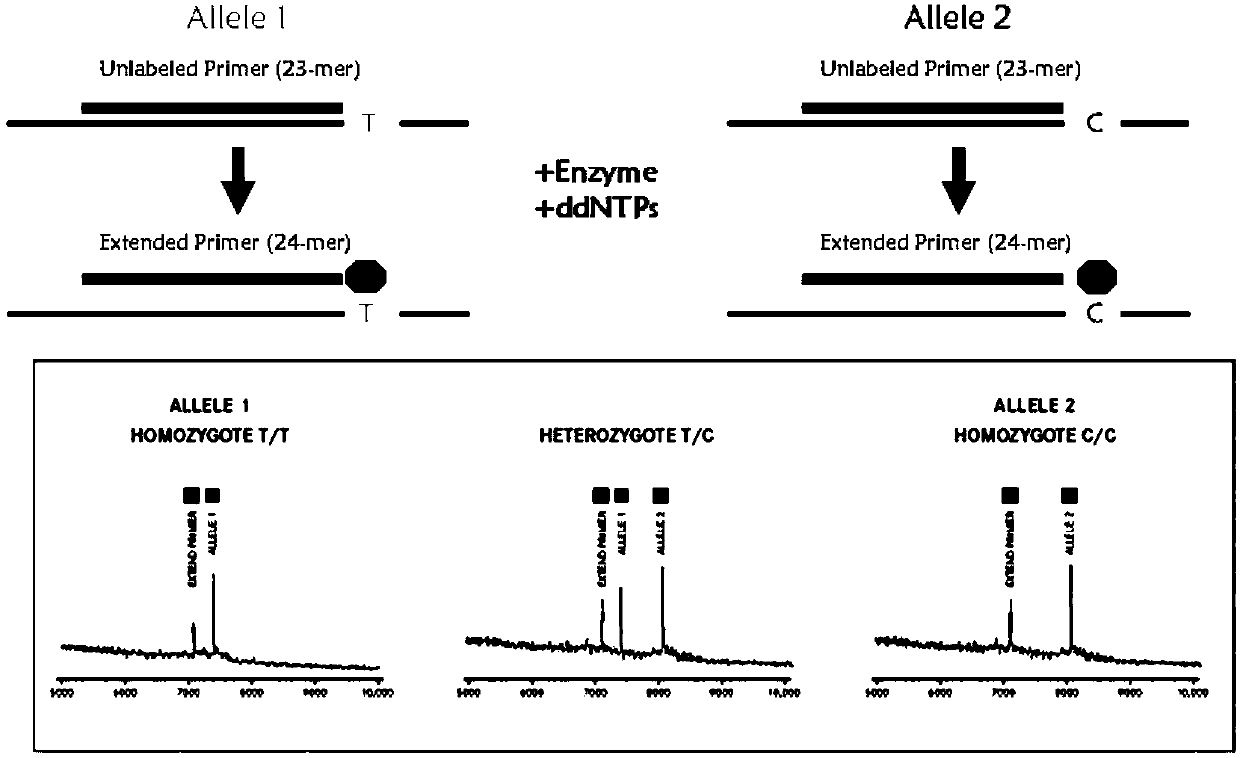
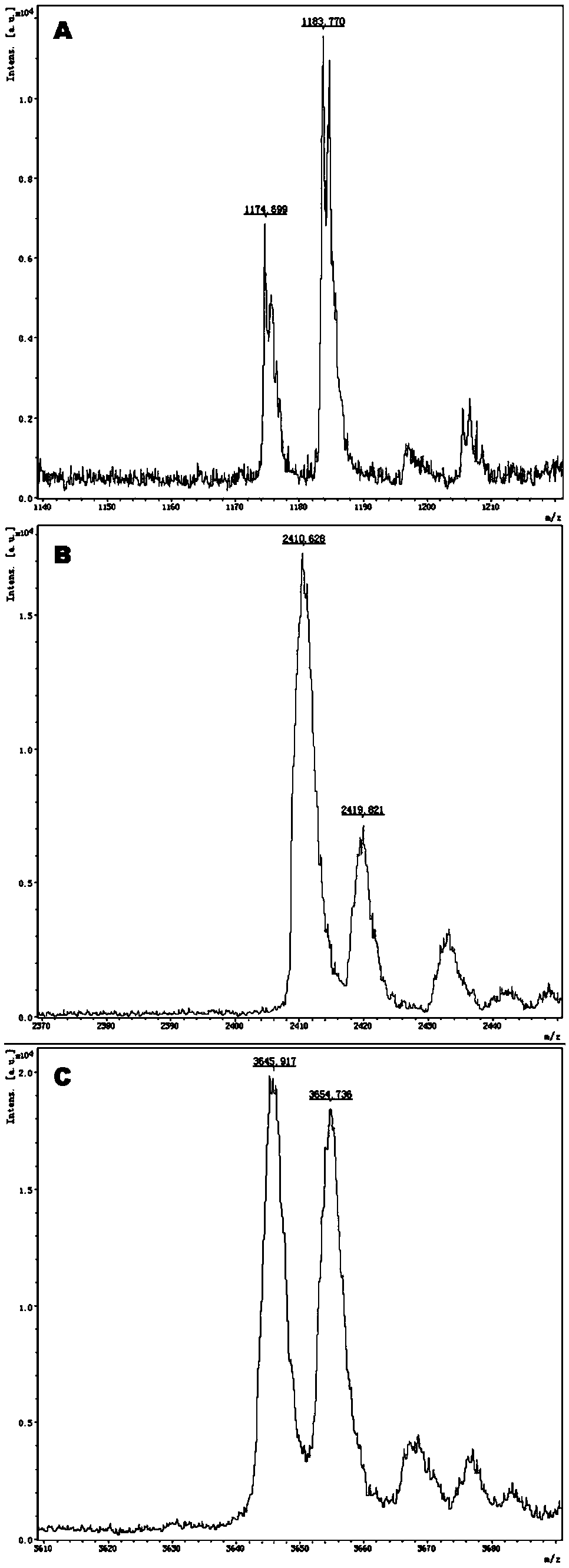
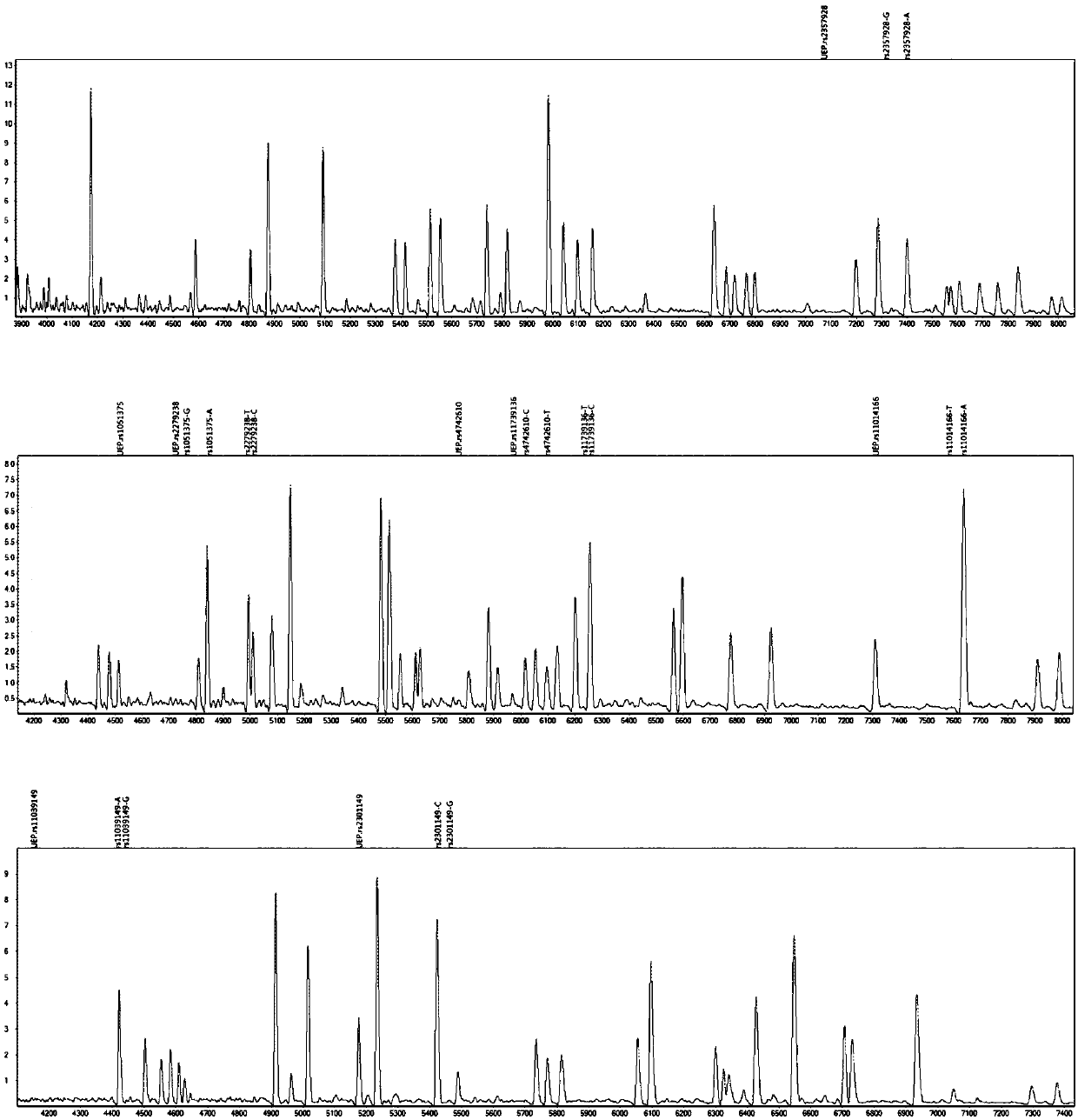
The invention provides a method and kit for guiding administration of verapamil by simultaneously detecting multiple loci related to the metabolism of the antihypertensive drug verapamil through matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) because different single nucleotide polymorphism (SNP) loci have extension primers with different molecular weights. The method includes the following steps: designing multiple amplification primers and extension primers according to eight target SNP loci to be detected; preparing a multiple amplification primerreaction system and an extension primer reaction system; in the reaction systems, simultaneously performing amplification and single-base extension reactions on the eight target SNP loci by using multiple sets of primers; and performing time-of-flight mass spectrometry analysis on the products after the single-base extension reactions, and identifying the genotypes of different drug metabolism-related SNP loci according to the products of different-molecular-weight extension primers represented by mass spectrum peaks to guide the administration of the antihypertensive drug verapamil. At the same time, the invention provides the detection kit using the method. The method can simultaneously detect the eight SNP loci related to the metabolism of the antihypertensive drug verapamil, and has the advantages of low costs, no need for synthesis of probes, short time consumption, simple and convenient result analysis, and extremely wide application fields.
Owner:BIOYONG TECH
Method for identifying individualized administration of verapamil by performing mass spectrometry through detection products
InactiveCN111172268AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationDrug metabolismMass Spectrometry-Mass Spectrometry
The invention provides a method for identifying individualized administration of verapamil by performing mass spectrometry through detection products. The method comprises the following steps: designing multiple amplification primers and extension primers according to eight target SNP loci to be detected; preparing a multiple amplification primer reaction system and an extension primer reaction system; in the reaction systems, simultaneously performing amplification and single-base extension reactions on the eight target SNP loci by using multiple sets of primers; and performing time-of-flightmass spectrometry analysis on the products after the single-base extension reactions, and identifying the genotypes of different drug metabolism-related SNP loci according to the products of different-molecular-weight extension primers represented by mass spectrum peaks to guide the administration of the antihypertensive drug verapamil. The method can simultaneously detect the eight SNP loci related to the metabolism of the antihypertensive drug verapamil, and has the advantages of low costs, no need for synthesis of probes, short time consumption, simple and convenient result analysis, and extremely wide application fields.
Owner:BIOYONG TECH
Verapamil hydrochloride film-controlled slow-release pellet capsule
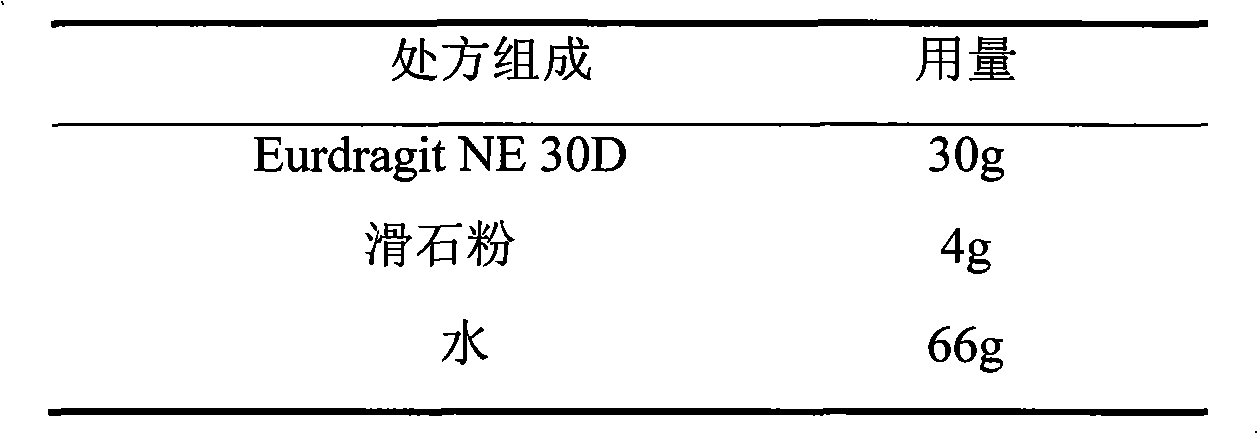
ActiveCN103211797AImprove permeabilityReduce permeabilityPharmaceutical non-active ingredientsGranular deliveryVerapamil HydrochlorideExcipient
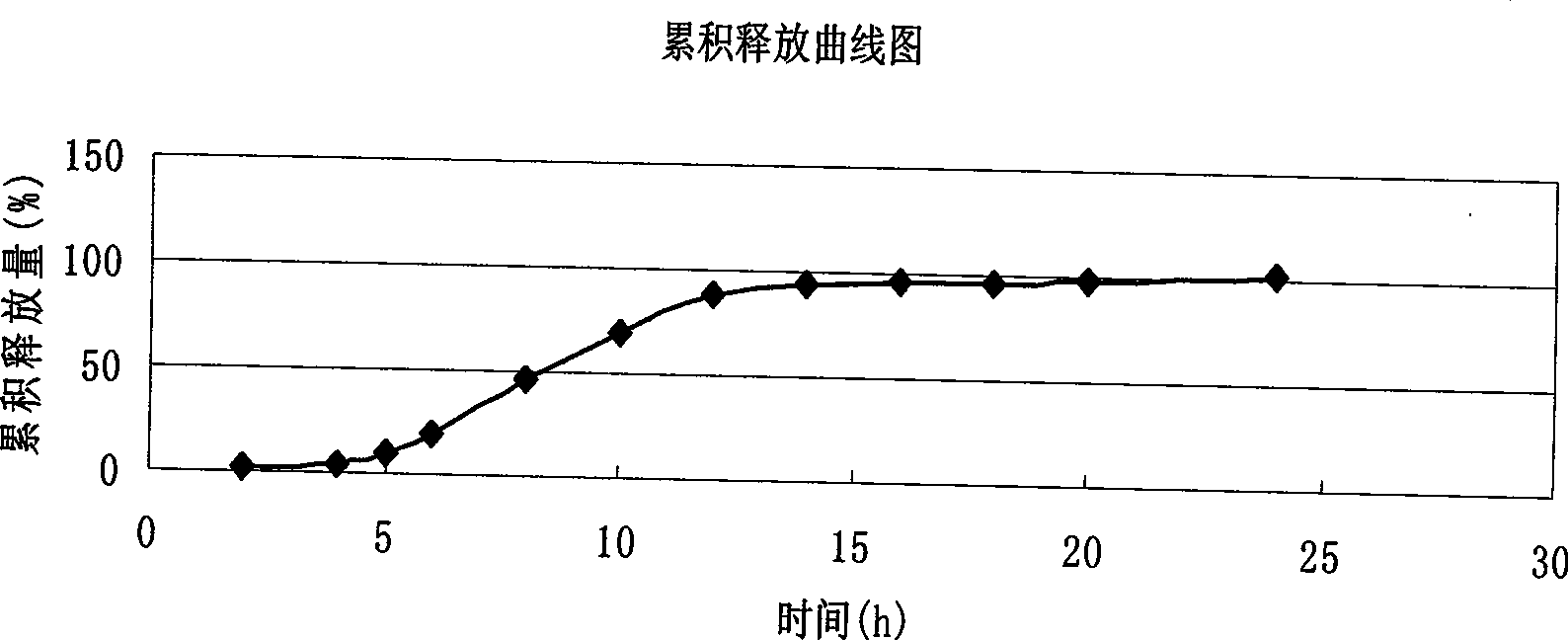
The invention relates to a verapamil hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the verapamil hydrochloride film-controlled slow-release pellet utilizes Eurdragit NE30D as a film-formation material. A pellet core of the verapamil hydrochloride film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit NE30D and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE30D to talcum powder is 30: 4 and a film weight increasing ratio is in a range of 19 to 35%. The verapamil hydrochloride film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the verapamil hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the verapamil hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Semen euphorbiae diterpene derivatives with MDR reversal activity and application of Semen euphorbiaediterpene derivatives
ActiveCN113277934AExcellent reversal activityOrganic chemistry methodsAntineoplastic agentsPerylene derivativesEuphorbia
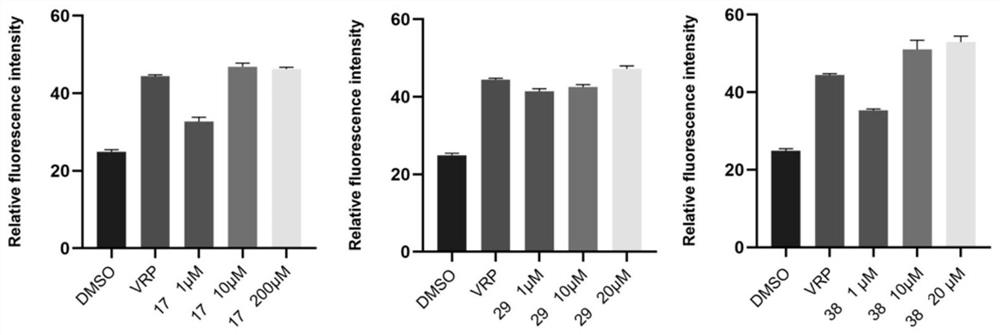
The invention belongs to the field of organic chemistry, and particularly relates to Semen euphorbiae diterpene derivatives with MDR reversal activity and application of the Semen euphorbiae diterpene derivatives. According to the invention, the novel Semen euphorbiae diterpene derivatives are extracted from euphorbia plant Semen euphorbiae, and are subjected to structural modification, a series of compounds are designed and synthesized, and the reversal activity in MCF-7 / ADR cells is superior to that of verapamil, wherein the reversal multiple of the compound 17 is up to 460.32.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Verapamil liposome and preparing method thereof
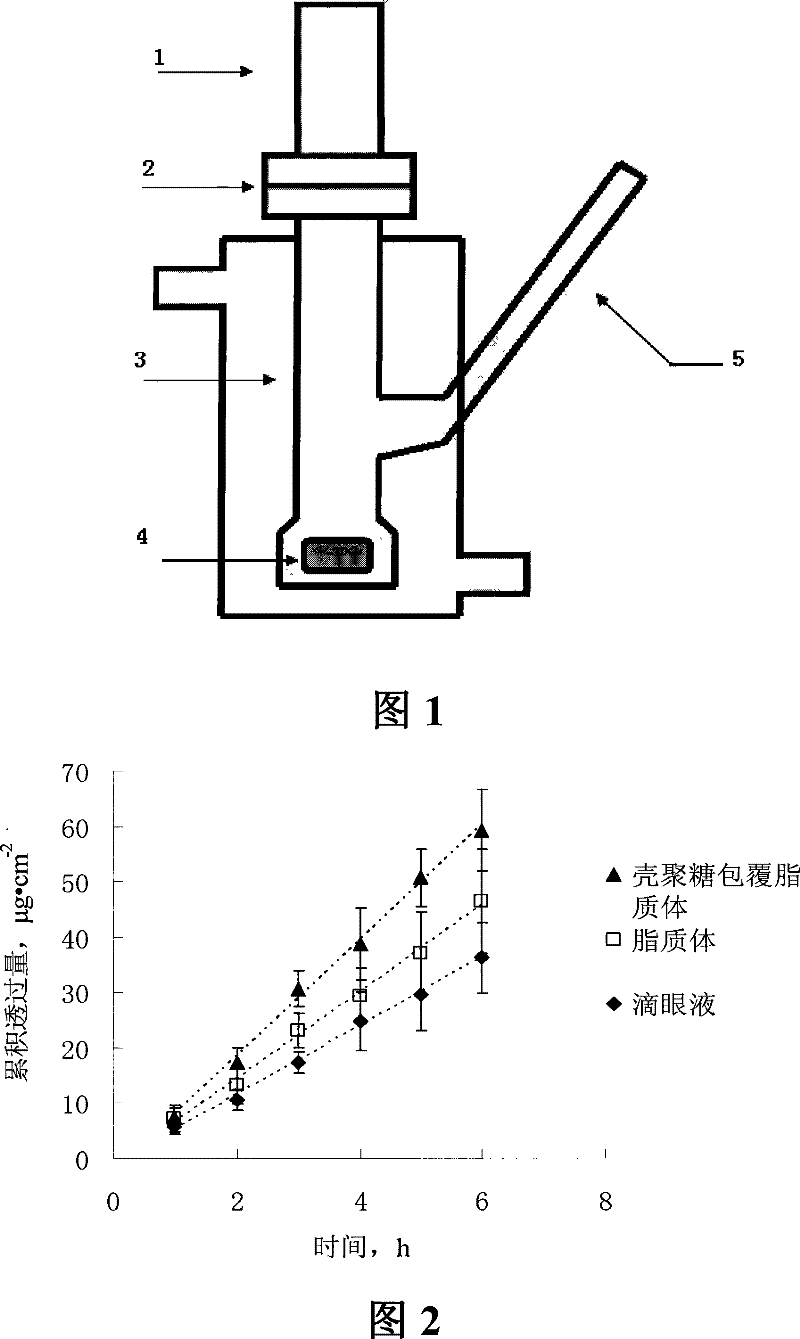
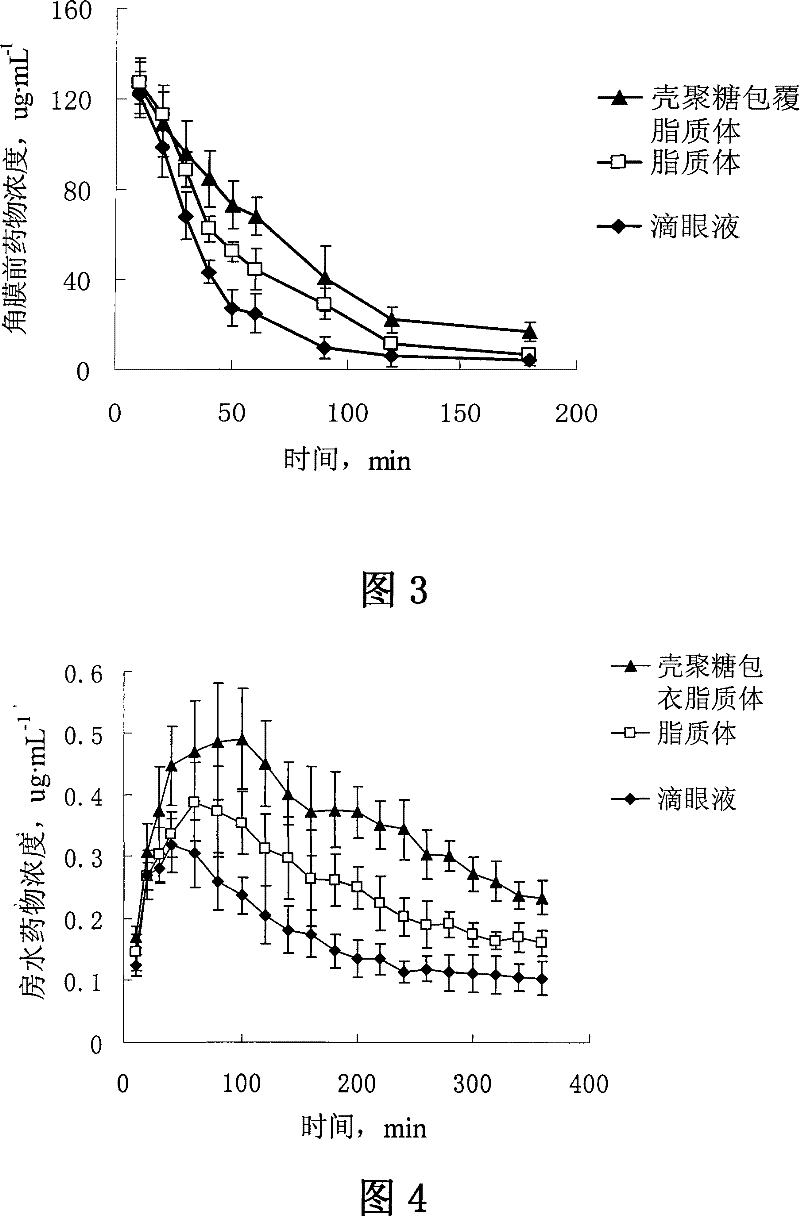
InactiveCN101199505BIncrease corneal penetrationImprove bioavailabilityPowder deliverySenses disorderEthanol InjectionCholesterol
The invention discloses verapamil liposome and a preparation method of the verapamil liposome, belonging to the technical field of medicine. The verapamil liposome is composed of verapamil, phospholipid, cholesterol, chitosan, cationic lipid and antioxidant, etc., all of which are in certain weight percentage. The verapamil liposome is prepared by adopting ammonium sulphate gradients method, filmhydration method, ethanol injection method and reverse phase evaporation method. The invention adopts chitosan or derivatives of the chitosan to modify the surface of the liposome, and applies the chitosan to ophthalmic preparation, which can further increase corneal retention of the liposome and can remarkably improve the corneal penetration amount of the drug, with obvious advantages compared with ordinary liposome. With high entrapment efficiency, good stability, good biocompatibility, biological adhesiveness and biodegradability, the prepared liposome can realize sustained release and long-acting administration, particularly suitable for administration on ocular region, and can better play the ocular effects of anti-glaucoma and anti-cataract of verapamil.
Owner:SHENYANG PHARMA UNIVERSITY
Primer combination for differentiating verapamil individualized medication types
InactiveCN111172265AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementDNA/RNA fragmentationHypertension medicationsMultiplex
The invention provides a primer combination for differentiating verapamil individualized medication types. An extension primer which has different molecular weights at different single nucleotide polymorphism (SNP) sites is utilized, so that through matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS), a plurality of sites pertinent to metabolism of a hypertension reducing medicine namely verapamil can be detected at the same time, and finally the primer combination for differentiating verapamil individualized medication types is obtained. A preparation method of the primer combination comprises the steps of according to 8 objective SNP sites to be detected, respectively designing a multiplex amplification primer and an extension primer; compounding amultiplex amplification primer reaction system and an extension primer reaction system; in the reaction systems, performing amplification and a single-base extension reaction on the 8 objective SNP sites at the same time with multiple sets of primers; and performing time-of-flight mass spectrometry analysis on products after the single-base extension reaction, identifying the genotypes of different drug metabolism pertinent SNPs according to products of different molecular weight extension primers represented by mass spectrum peaks, and guiding the medication of the hypertension reducing medicine namely the verapamil. The primer combination disclosed by the invention can detect 8 metabolism pertinent SNP sites of the hypertension reducing medicine namely the verapamil at the same time, and has the advantages of being low in cost, free from synthetized probes, short in time consumption, simple and convenient in result analysis, and extremely broad in application field.
Owner:BIOYONG TECH
Insecticidal composition and application thereof
The invention relates to the field of insecticides and provides an insecticidal composition and application thereof. The insecticidal composition provided by the invention comprises an abamectin pesticide and a specific P-glycoprotein inhibitor, wherein the specific P-glycoprotein inhibitor is preferably verapamil. According to the invention, the insecticidal activity of the abamectin pesticide isenhanced, the usage amount of the field pesticide can be properly reduced, and the possible problem of pesticide residues is avoided; a synergist is environment-friendly and does not damage an ecological system; due to the use of the synergist, the sensitivity of prodenia litura to the abamectin pesticide can be remarkably improved; on the premise that poisoning effect is not changed, the use amount of the abamectin pesticide can be properly reduced; and the dosage of the insecticidal composition be saved, the pesticide effect of original insecticides can be improved, the pressure of the insecticidal composition to the environment can be reduced and the resistance of pests to the insecticidal composition can be delayed.
Owner:AGRI GENOME INST OF SHENZHEN CHINESE ACADEMY OF AGRI SCI
Application of hematoporphyrin derivative combined with chemical drug in breast cancer treatment
PendingCN112675167AGood reversal effectOrganic active ingredientsOrganic chemistryChemotherapeutic drugsPharmaceutical drug
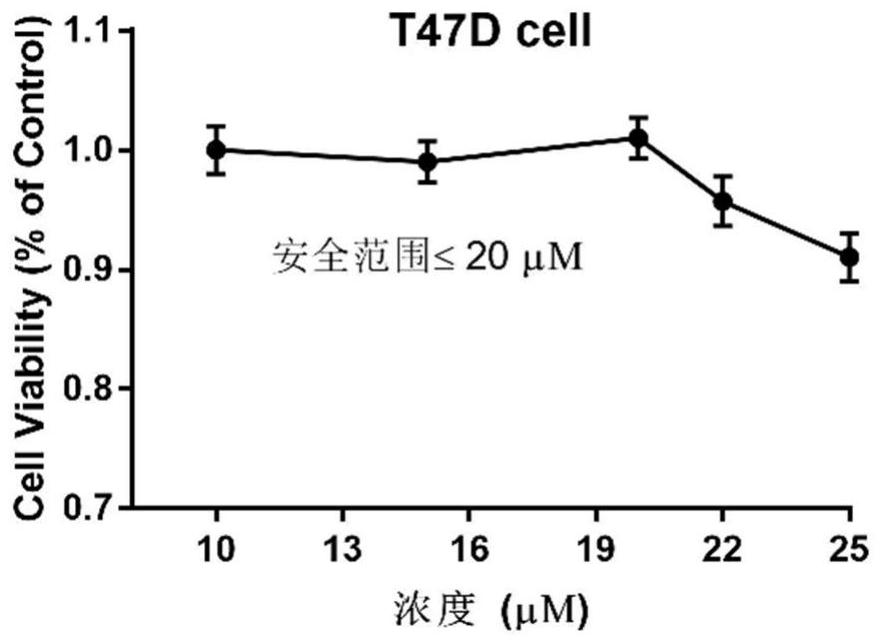
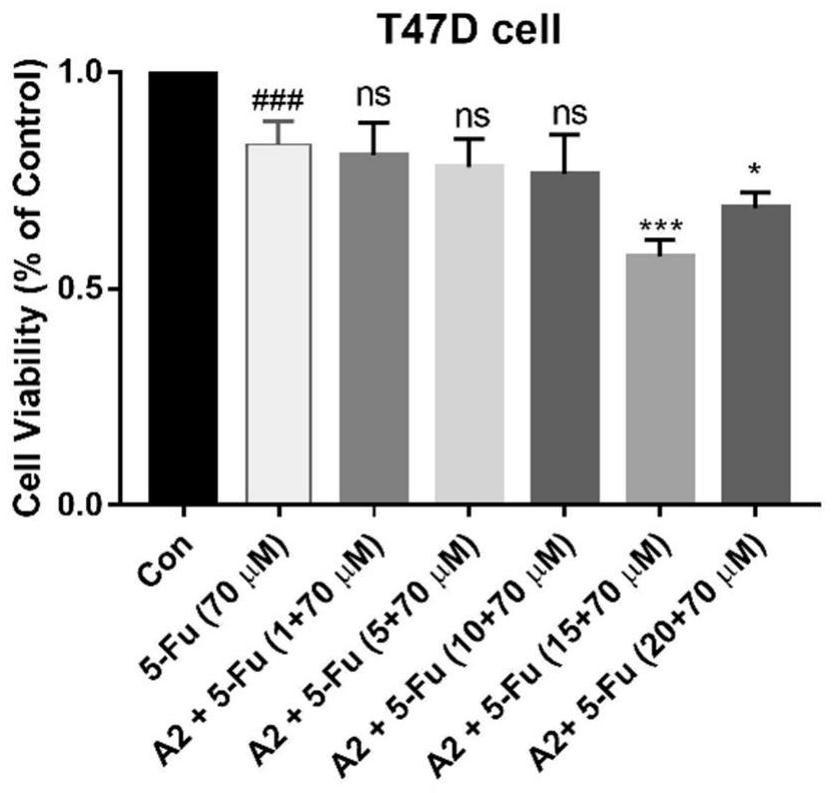
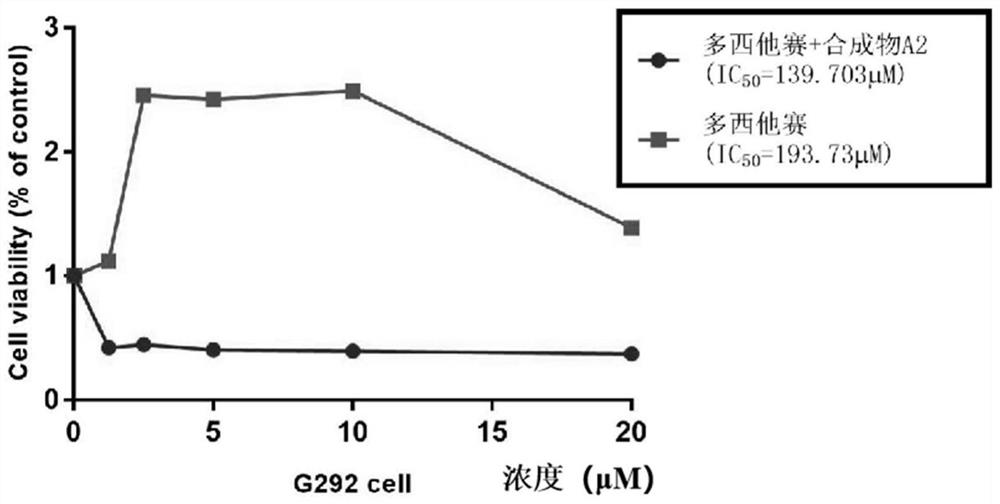
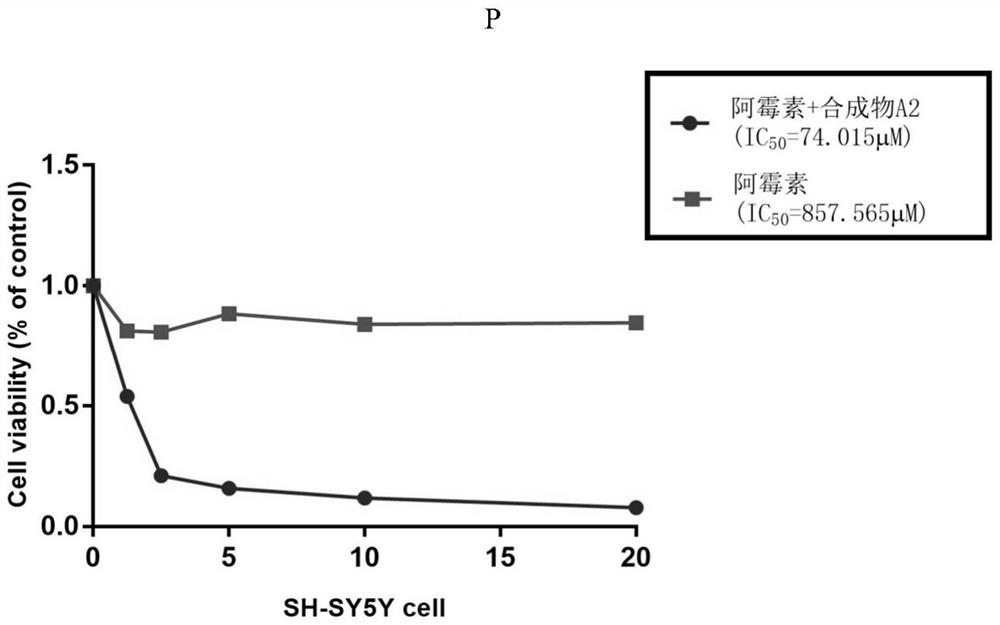
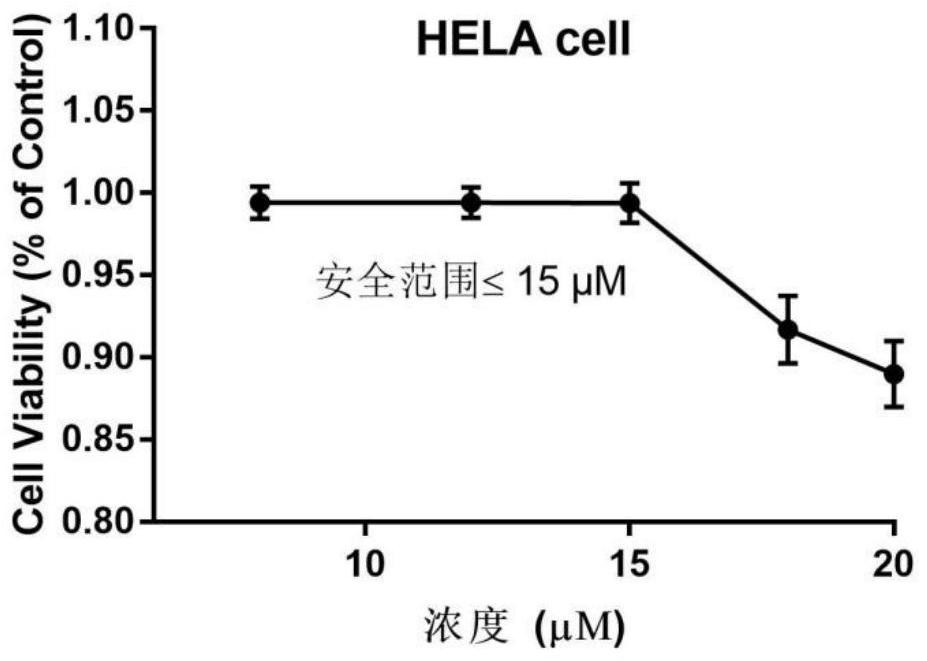
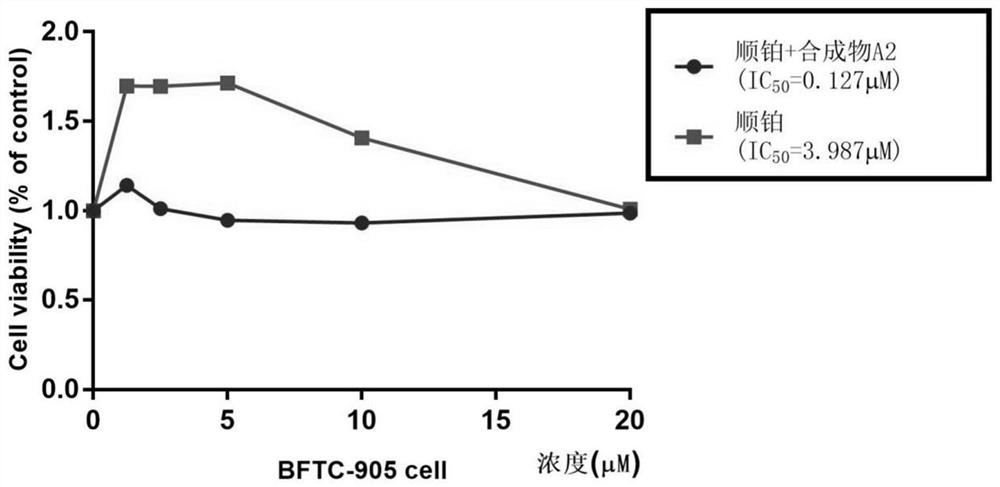
The invention belongs to the field of medicine and pharmacology for increasing sensitivity of chemotherapeutic drugs, and particularly relates to application of a hematoporphyrin-verapamil fragment hematoporphyrin derivative A2 in tolerance reversing and sensitization of breast cancer chemotherapeutic drugs. The hematoporphyrin derivative A2 has different degrees of sensitization of the chemotherapeutic drugs for inhibiting breast cancer cell proliferation, which indicates that the hematoporphyrin derivative A2 can be developed into a sensitizer or a reverse drug-resistant agent of the chemotherapeutic drugs and used for treating breast cancer patients.
Owner:范平生 +5
Application of marine phospholipids as active ingredients in the preparation of drugs for preventing and/or treating heart disease
ActiveCN112494499BImprove protectionGood treatment effectOrganic active ingredientsCrustacean material medical ingredientsDiseaseThrombus
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Application of hematoporphyrin derivative combined with chemical drug in treatment of osteosarcoma
PendingCN112516314AGood reversal effectOrganic active ingredientsEnergy modified materialsChemotherapeutic drugsChemical compound
The invention belongs to the field of medicine and pharmacology for improving chemotherapy drug sensitivity, and particularly relates to application of a hematoporphyrin-verapamil fragment compound A2in reversing the tolerance and sensitization of chemotherapy drugs for osteosarcoma. Different degrees of the sensitization chemotherapeutic drugs of the compound A2 inhibit the proliferation of osteosarcoma cells, which indicates that the compound A2 can be developed into a sensitizer or a reverse drug resistance of the chemotherapeutic drugs and is used for treating osteosarcoma patients.
Owner:范平生 +5
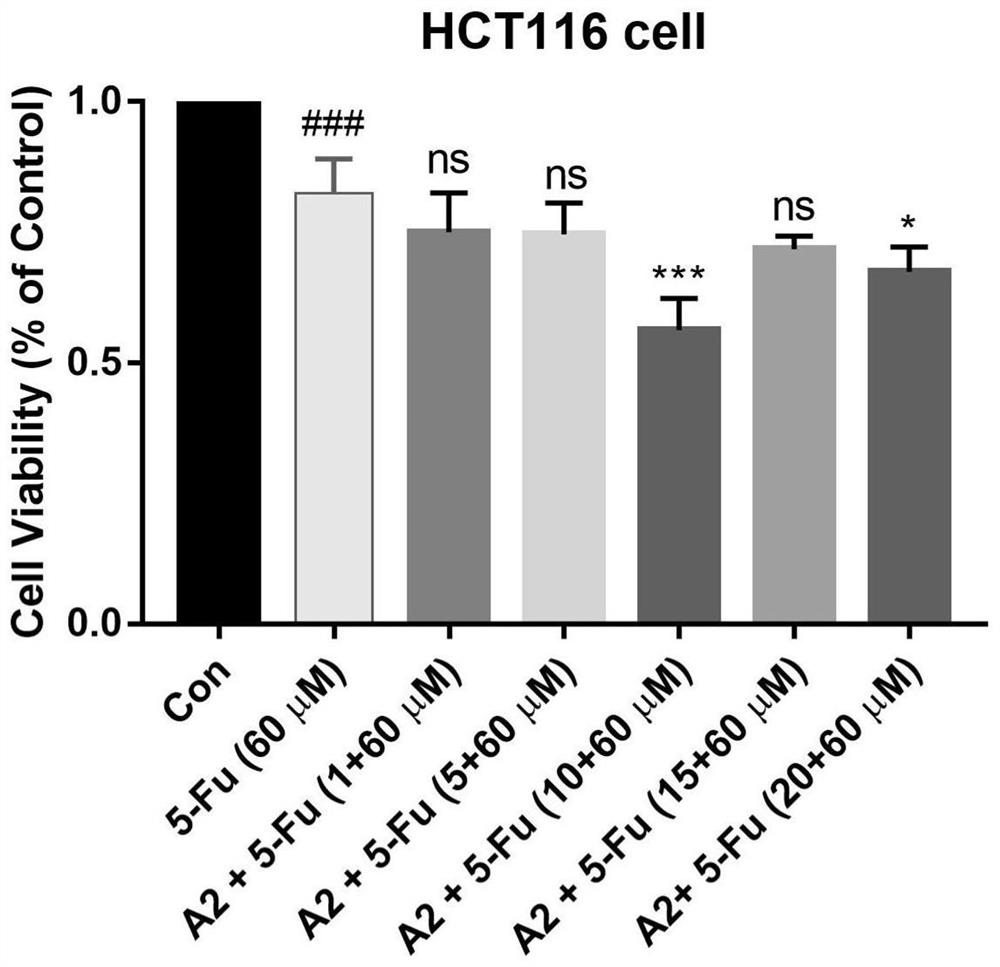
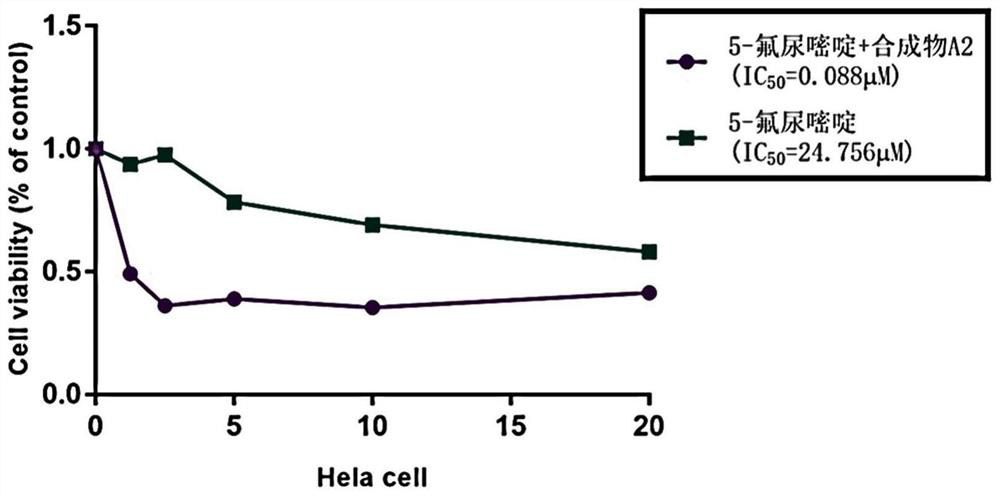
Application of combination of hematoporphyrin derivative and chemical drug in colon cancer treatment
PendingCN112516313AGood reversal effectEnergy modified materialsPharmaceutical delivery mechanismChemotherapeutic drugsPharmaceutical drug
The invention belongs to the field of medicines for improving the sensitivity of chemotherapeutic drugs, and particularly relates to application of a hematoporphyrin-verapamil fragment hematoporphyrinderivative A2 in tolerance reversion and sensitivity improvement of a colon cancer chemotherapeutic drug. The hematoporphyrin derivative A2 has the effect of improving the sensitivity of the chemotherapeutic drug for inhibiting the proliferation of colon cancer cells to different degrees, and it is indicated that the hematoporphyrin derivative A2 can be developed into a sensitizer or a drug tolerance reversion agent of the chemotherapeutic drug and is used for treating patients with colon cancer.
Owner:范平生 +5
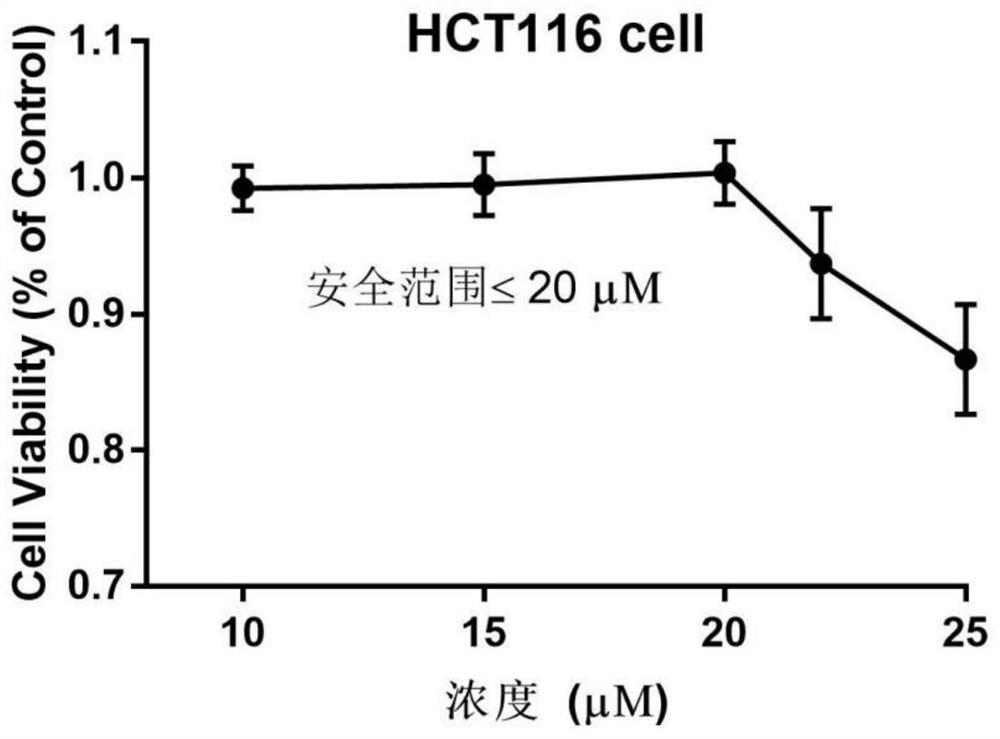
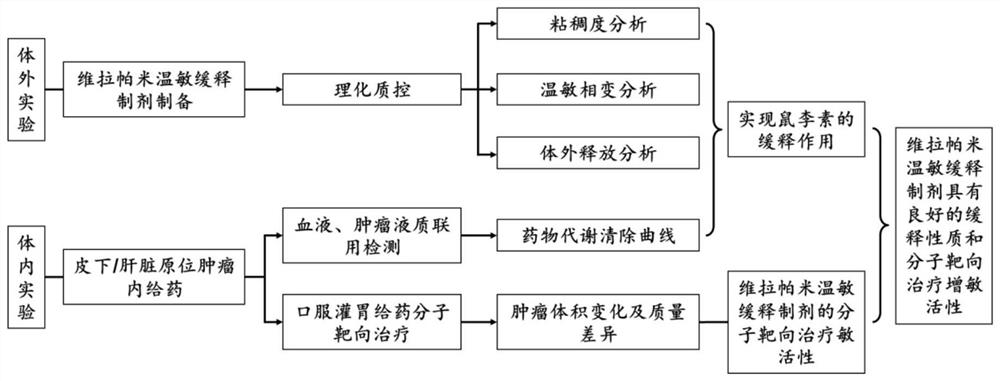
A kind of verapamil thermosensitive sustained-release preparation and its preparation method and application
ActiveCN111840255BIncrease viscosityPlay a protective roleMacromolecular non-active ingredientsAntineoplastic agentsPharmaceutical drugUnilamellar liposome
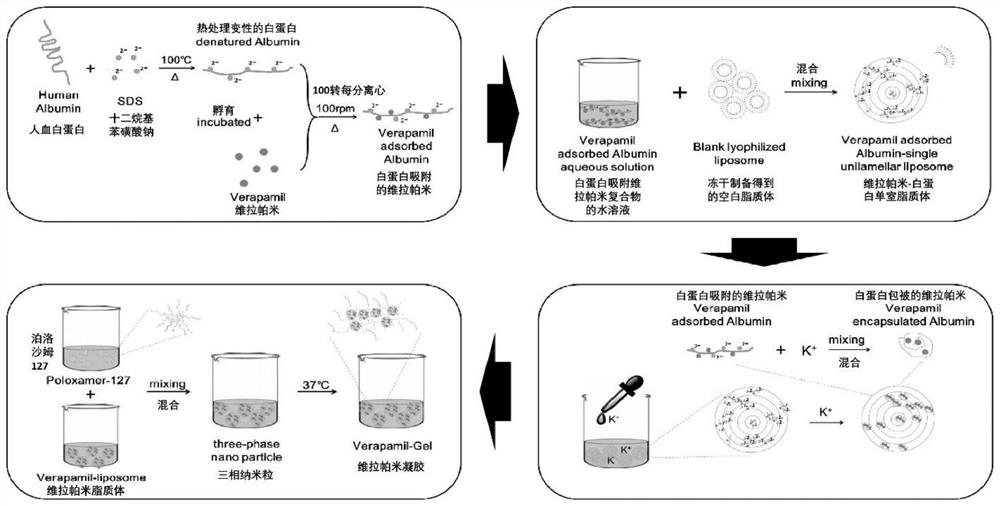
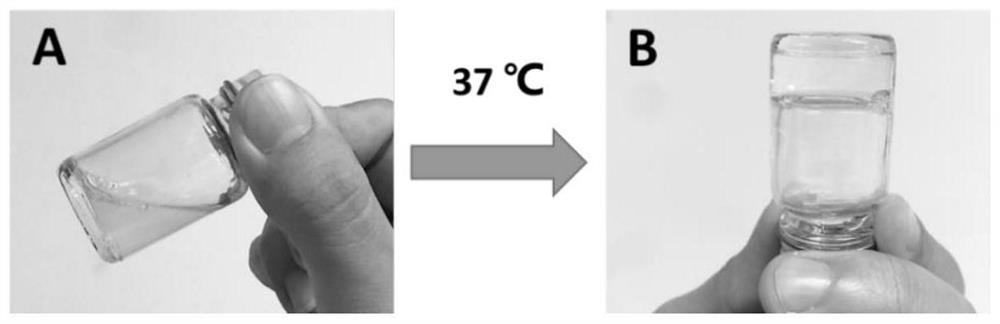
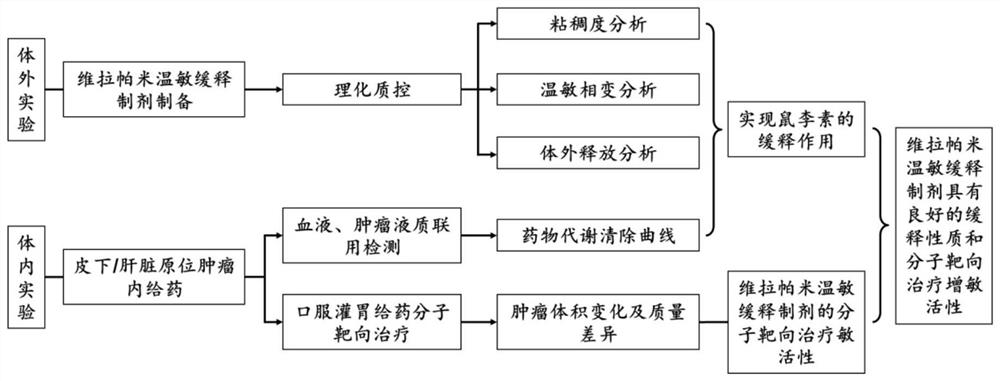
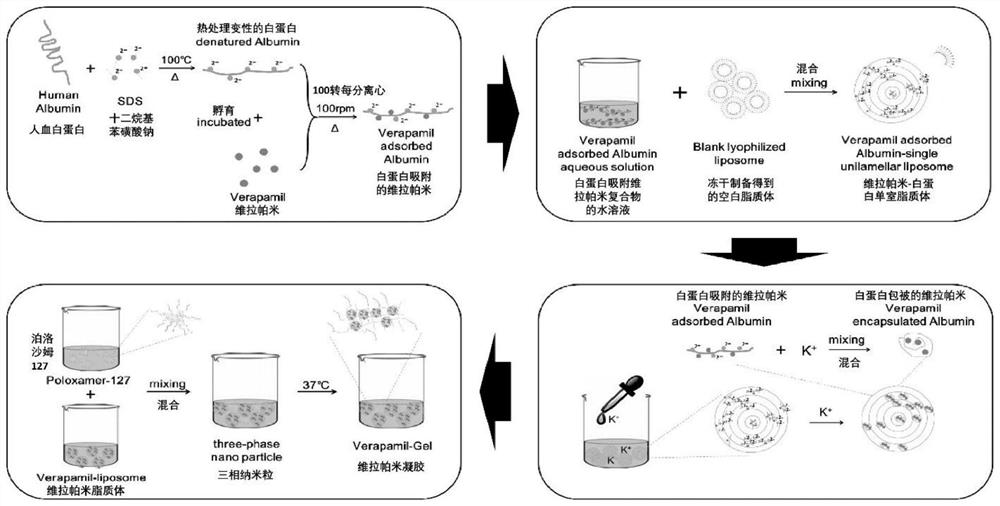
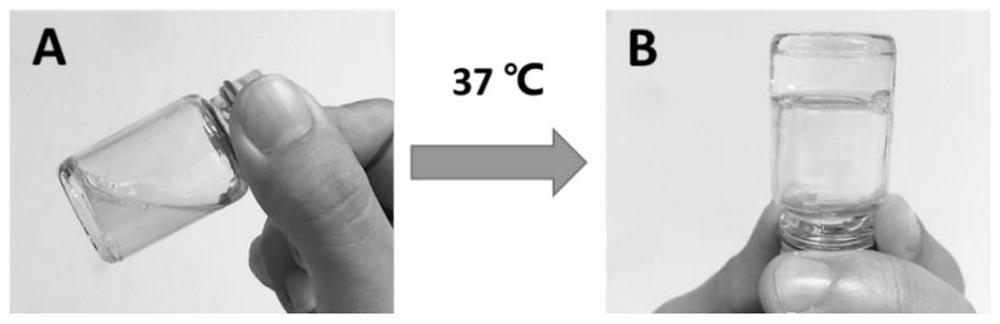
The invention provides a temperature-sensitive sustained-release preparation of verapamil, comprising: drug-loaded denatured albumin, multilamellar liposome (SUV) and thermosensitive glue; wherein the drug-loaded denatured albumin includes denatured albumin and an effective amount of verapamil. The present invention also provides a preparation method and application of the above-mentioned verapamil temperature-sensitive sustained-release preparation. The above preparation adopts denaturation of human serum albumin and incubates with verapamil to absorb verapamil to form drug nanoparticles, encapsulates albumin-verapamil drug nanoparticles with small unilamellar liposomes, and finally mixes with thermosensitive preparation Loxamer 127 is mixed to form a three-phase sustained-release system of drug-loaded albumin nanoparticles-multilamellar liposomes-thermosensitive adhesive.
Owner:北京丰帆生物医药科技有限公司
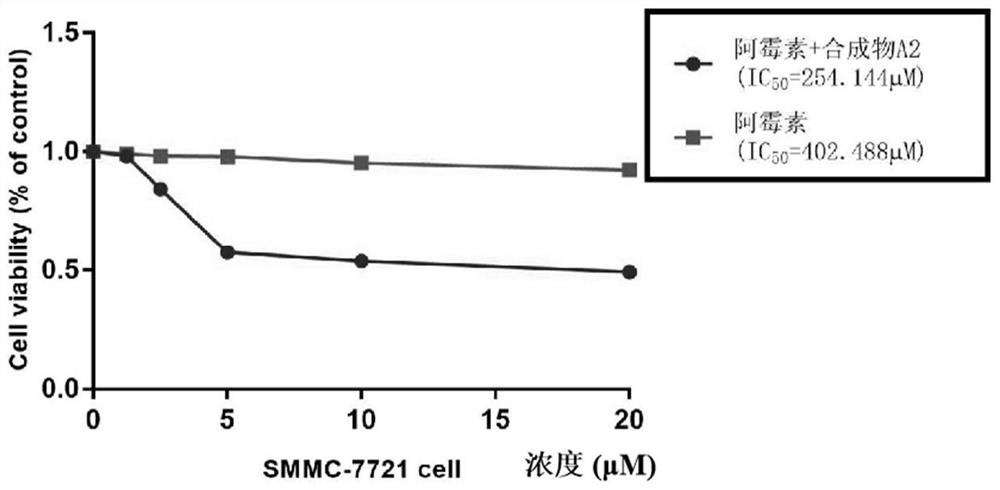
Application of hematoporphyrin derivative combined chemical drug in liver cancer treatment
PendingCN112494650AGood reversal effectOrganic active ingredientsEnergy modified materialsChemotherapeutic drugsPharmaceutical drug
The invention belongs to the field of medicine for increasing the sensitivity of chemotherapeutic drugs, and particularly relates to application of a hematoporphyrin derivative A2 of a hematoporphyrincombined verapamil fragment in tolerance and sensitization of liver cancer reverse chemotherapeutic drugs. The hematoporphyrin derivative A2 has the effect of inhibiting liver cancer cell proliferation of sensitization chemotherapeutic drugs of different degrees, and the hematoporphyrin derivative A2 can be developed into a sensitizer or a reverse drug-resistant agent of the chemotherapeutic drugs and used for treating liver cancer patients.
Owner:范平生 +5
Method for discriminating individualized verapamil medication by mass spectrometry through primer composition
InactiveCN111172264AQuality Medical ServicesPromoting the process of rational drug useMicrobiological testing/measurementMultiplexDrug metabolism
The invention provides a method for discriminating individualized verapamil medication by mass spectrometry through a primer composition. The method comprises the following steps: respectively designing multiple amplification primers and extension primers according to eight to-be-detected target SNP loci; preparing a multiplex amplification primer reaction system and an extension primer reaction system; in the reaction systems, simultaneously and respectively carrying out amplification and single-base extension reaction on the eight target SNP loci by using a plurality of sets of primers; andcarrying out time-of-flight mass spectrometry analysis on a product after the single base extension reaction, identifying genotypes of SNP related to different drug metabolism according to products ofextension primers with different molecular weights represented by a mass spectrum peak, and guiding the medication of hypotensive drug verapamil. The method can simultaneously detect eight SNP loci related to metabolism of the hypotensive drug verapamil, and has the advantages of low cost, no need of probe synthesis, short time consumption, simple and convenient result analysis and extremely wideapplication field.
Owner:BIOYONG TECH
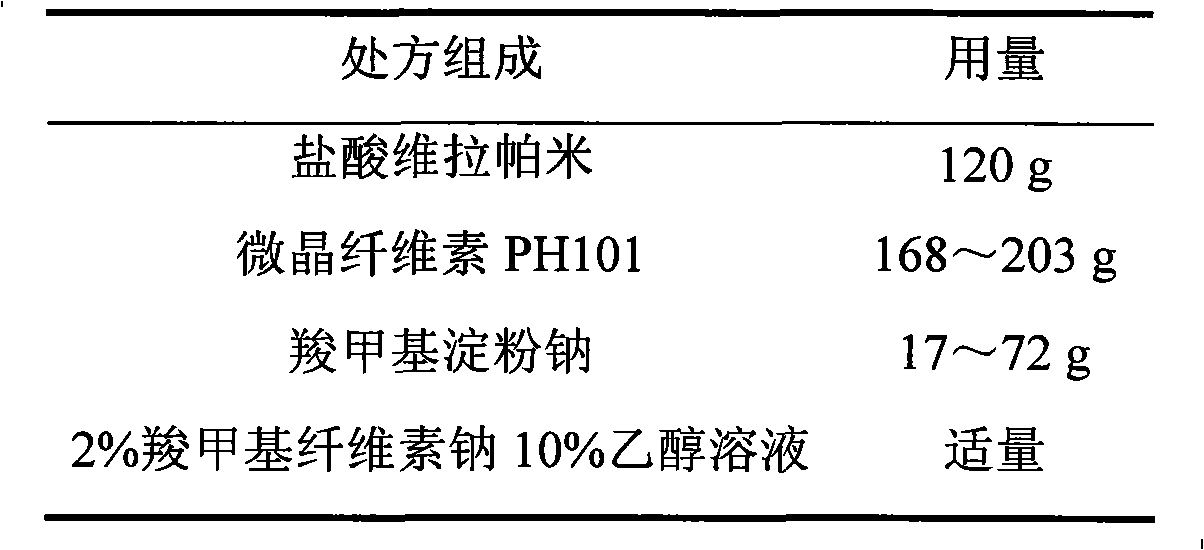
Preparation method of verapamil hydrochloride sustained-release preparation
InactiveCN108992395AGood slow release functionRelease stabilityPowder deliveryNervous disorderVerapamil HydrochloridePolyethylene glycol
The invention provides a preparation method of a verapamil hydrochloride sustained-release preparation. The preparation method comprises the following steps: dissolving verapamil hydrochloride in 95%ethanol to obtain a solution A, and adding hydroxypropyl beta-cyclodextrin and mannitol into the solution A to obtain a solution B; dissolving polylactic acid and polyethylene glycol 200 in acetone toobtain a solution C, mixing the solution B with the solution C to obtain a solution D, transferring the solution D to a magnetic stirrer, continuously stirring the solution D for 12 hours, lowering the temperature of the solution D to 0-1 DEG C within 2 hours, allowing to stand still for 12 hours, and maintaining the temperature of the solution D at 0-1 DEG C during the standing period; heating the solution D after standing till for 12 hours, continuously stirring when the temperature of the solution D is raised to 15-18 DEG C, controlling the temperature of the solution D at 15-18 DEG C whenstirring, and preparing the verapamil hydrochloride sustained-release preparation with a low-temperature spray drying method after continuously stirring for 12 hours. According to the invention, thedosage of a capsule wall material is moderate, the drying temperature of materials is low, and the prepared drug-loading preparation is uniform in size and stable in drug release, and has the characteristic of slow release.
Owner:刘丽
Application of hematoporphyrin derivative in treatment of stomach cancer together with chemical drug
PendingCN112546225AGood reversal effectOrganic active ingredientsEnergy modified materialsOncologyStomach cancer
The invention belongs to the field of chemotherapeutic drug sensitivity enhancing medicine and particularly relates to use of a hematoporphyrin-verapamil-fragment-combined hematoporphyrin derivative A2 in tolerance and sensitivity enhancing of stomach cancer reversing chemotherapeutic drugs. The hematoporphyrin derivative A2 has different extents of chemotherapeutic-drug-sensitivity-enhanced stomach cancer cell proliferation inhibiting actions, and it is shown that the hematoporphyrin derivative A2 can be developed into chemotherapeutic-drug sensitizing agents or drug resistance reversing agents and is applied to treatment on stomach cancer sufferers.
Owner:范平生 +5
Application of marine phospholipid as effective component in preparation of medicine for preventing and/or treating heart diseases
ActiveCN112494499AImprove protectionGood treatment effectOrganic active ingredientsCrustacean material medical ingredientsVerapamilumRat heart
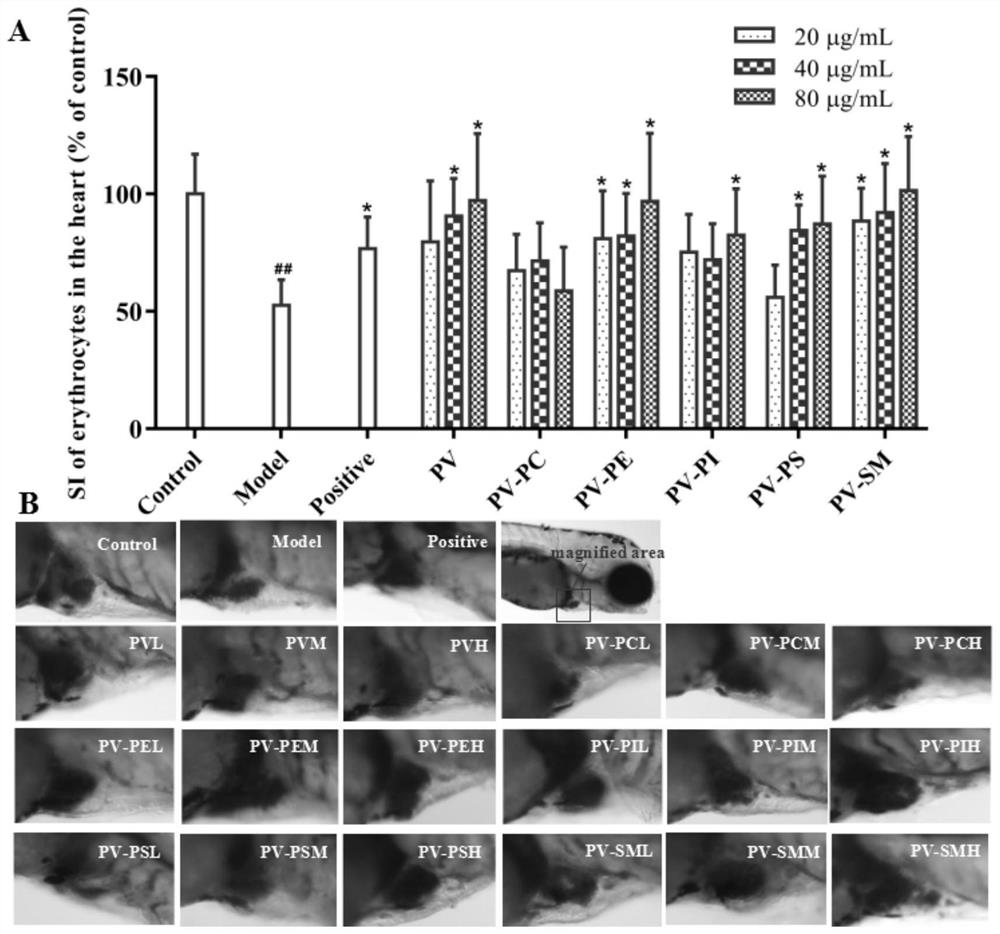
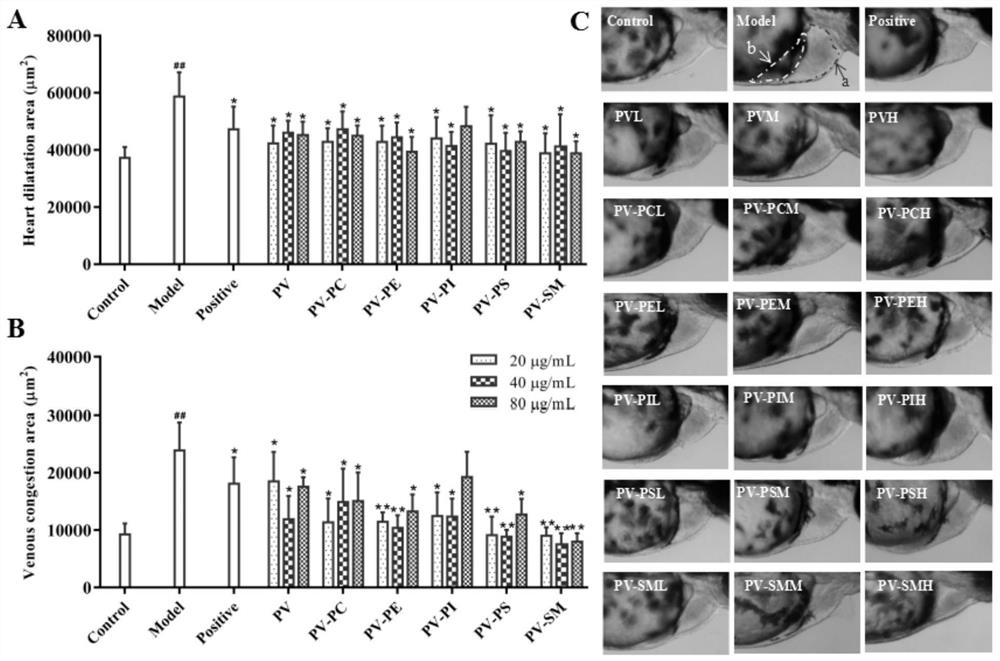
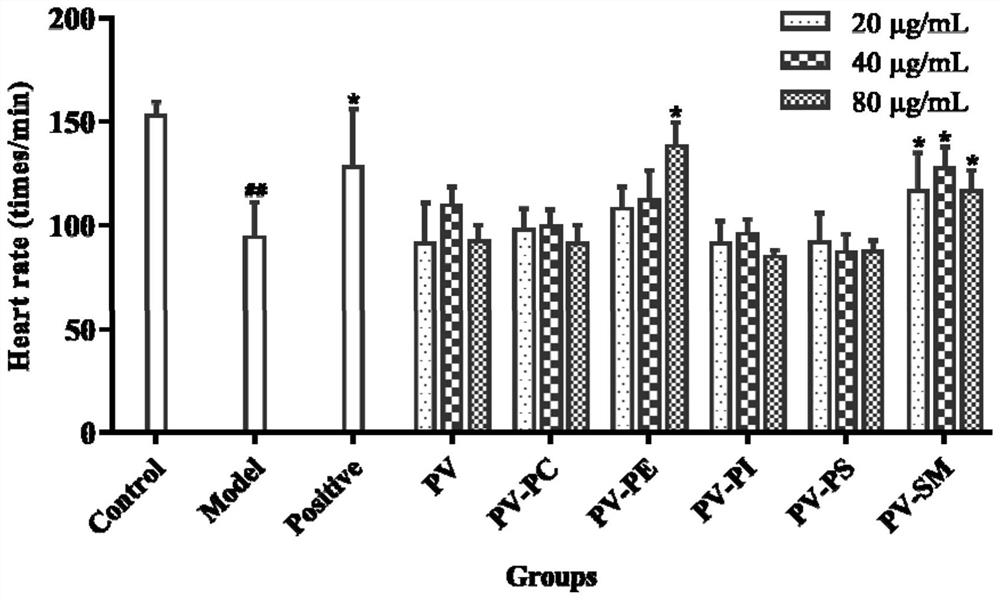
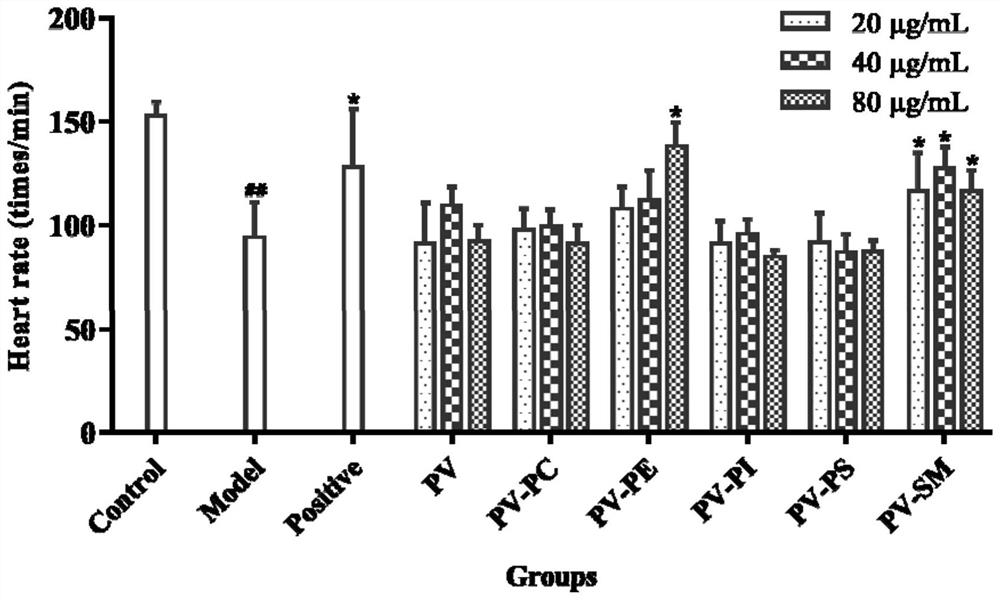
The invention relates to application of marine phospholipid as an effective component in preparation of a medicine for preventing and / or treating heart diseases. The marine phospholipid is separated to obtain five phospholipid components comprising phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine and sphingomyelin. It is found that the components have good protection and treatment effects on the heart; and the phospholipid components have a remarkable heart protection effect on arachidonic acid-induced zebra fish thrombus, verapamil-induced zebra fish heart failure and terfenadine-induced zebra fish arrhythmia, and can obviously reduce zebra fish congestion and cardiac dilatation and promote heart rate to tend to be normal.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Verapamil temperature-sensitive sustained-release preparation and preparation method and application thereof
ActiveCN111840255AIncrease viscosityPlay a protective roleMacromolecular non-active ingredientsAntineoplastic agentsAdhesivePharmaceutical drug
The invention provides a verapamil temperature-sensitive sustained-release preparation. The verapamil temperature-sensitive sustained-release preparation comprises drug-loaded denatured albumin, a multi-chamber liposome (SUV) and a temperature-sensitive adhesive, wherein the drug-loaded denatured albumin comprises denatured albumin and an effective amount of verapamil. The invention also providesa preparation method and application of the verapamil temperature-sensitive sustained-release preparation. According to the preparation, human serum albumin is denatured and then incubated with verapamil to adsorb verapamil to form drug nanoparticles, albumin-verapamil drug nanoparticles are wrapped with small single-chamber lipidosome, and finally the drug nanoparticles are mixed with a temperature-sensitive preparation poloxamer 127 to form a drug-loaded albumin nanoparticle-multi-chamber lipidosome-temperature-sensitive adhesive three-phase slow release system.
Owner:北京丰帆生物医药科技有限公司
Application of verapamil hydrochloride in the preparation of fungicides for preventing and treating plant diseases caused by plant pathogenic bacteria
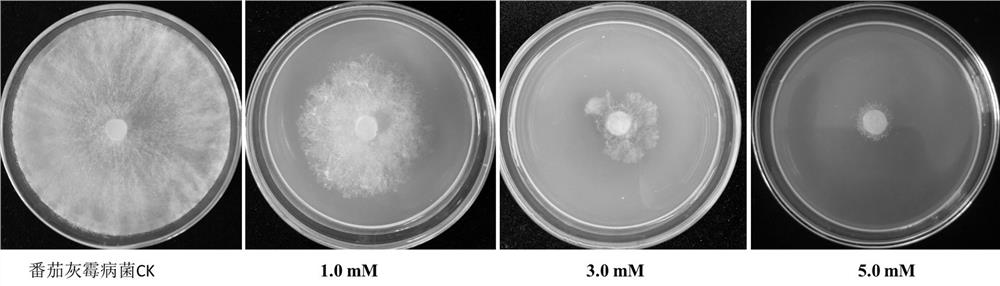
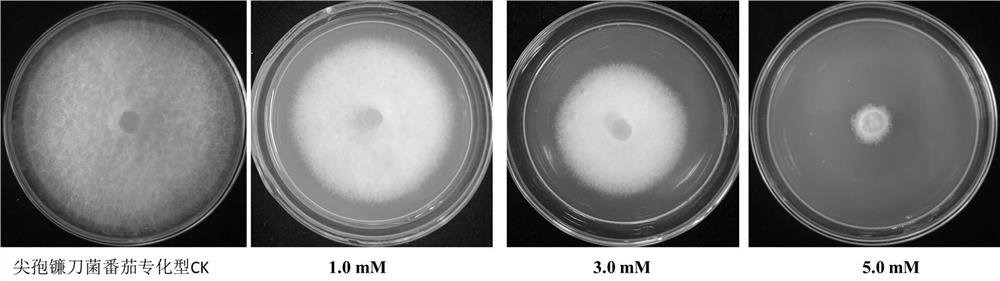
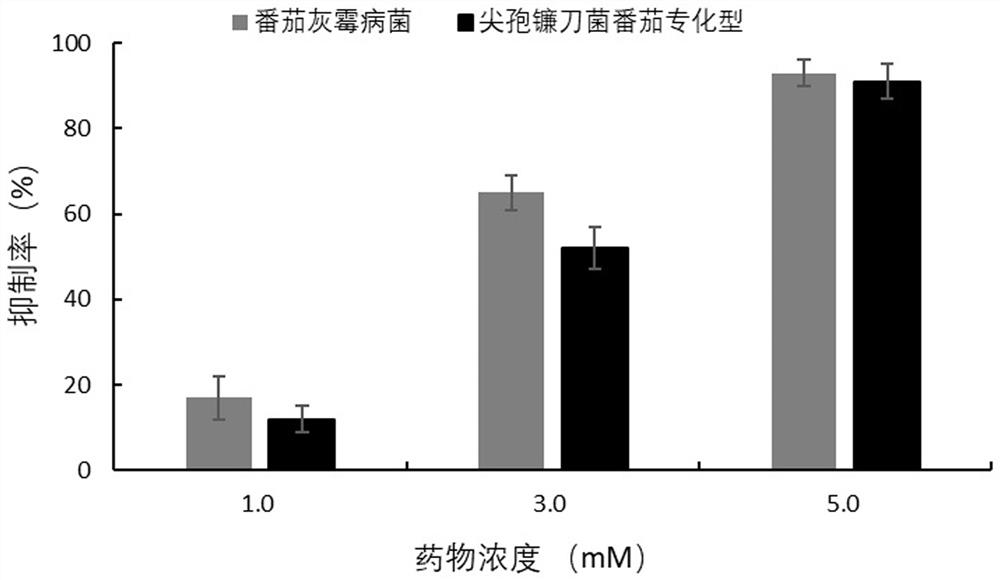
The invention provides the application of verapamil hydrochloride in the preparation of fungicides for preventing and treating plant diseases caused by plant pathogenic bacteria. The present invention proves that verapamil hydrochloride has the effect on tomato Botrytis cinerea and Fusarium oxysporum through experiments on mycelium growth, spore germination and germ tube elongation of tomato Botrytis cinerea and Fusarium oxysporum. The tomato specialized type has strong inhibitory effect, and it can be prepared as a fungicide for preventing and treating botrytis cinerea and plant diseases caused by Fusarium oxysporum. Traditional pesticides pollute the environment with high residues and threaten food safety. The use of a large number of pesticides also leads to the enhancement of the resistance of pathogenic bacteria. As a fungicide, verapamil hydrochloride has the advantages of high efficiency and low toxicity. It is suitable for the requirements of chemical control of plant diseases and can ensure the high quality of agricultural products and fruits and vegetables. Development requirements, with broad research and market prospects.
Owner:QINGDAO AGRI UNIV
Application of haematoporphyrin derivative to treatment of neuroblastoma
PendingCN112618725AGood reversal effectPharmaceutical non-active ingredientsAntineoplastic agentsPharmaceutical drugOncology
The invention belongs to the field of medicine and pharmacology for sensitizing chemotherapy drug, in particular to application of a haematoporphyrin-verapamil fragment compound A2 to tolerance reversing and sensitization of chemotherapy drugs for neuroblastoma. The compound A2 sensitizes the chemotherapy drugs in different degrees to inhibit cell proliferation of the neuroblastoma, which indicates that the compound A2 can be developed into a sensitizer or a drug resistance reversing agent of the chemotherapy drugs for treating a patient with the neuroblastoma.
Owner:范平生 +5
Application of hematoporphyrin derivative combined with chemical drug in cervical cancer treatment
PendingCN112451668AGood reversal effectOrganic active ingredientsEnergy modified materialsChemotherapeutic drugsCervical ca
The invention belongs to the field of medicine and pharmacology for improving the sensitivity of chemotherapeutic drugs, and particularly relates to application of a hematoporphyrin-verapamil fragmenthematoporphyrin derivative A2 in reversing tolerance and sensitization of cervical cancer chemotherapeutic drugs. The hematoporphyrin derivative A2 has the effect of sensitive chemotherapeutics at different degrees for inhibiting proliferation of cervical cancer cells, which indicates that the hematoporphyrin derivative A2 can be developed into a sensitizer or a reversal resistance agent of the chemotherapeutics for treating patients with cervical cancer.
Owner:范平生 +5
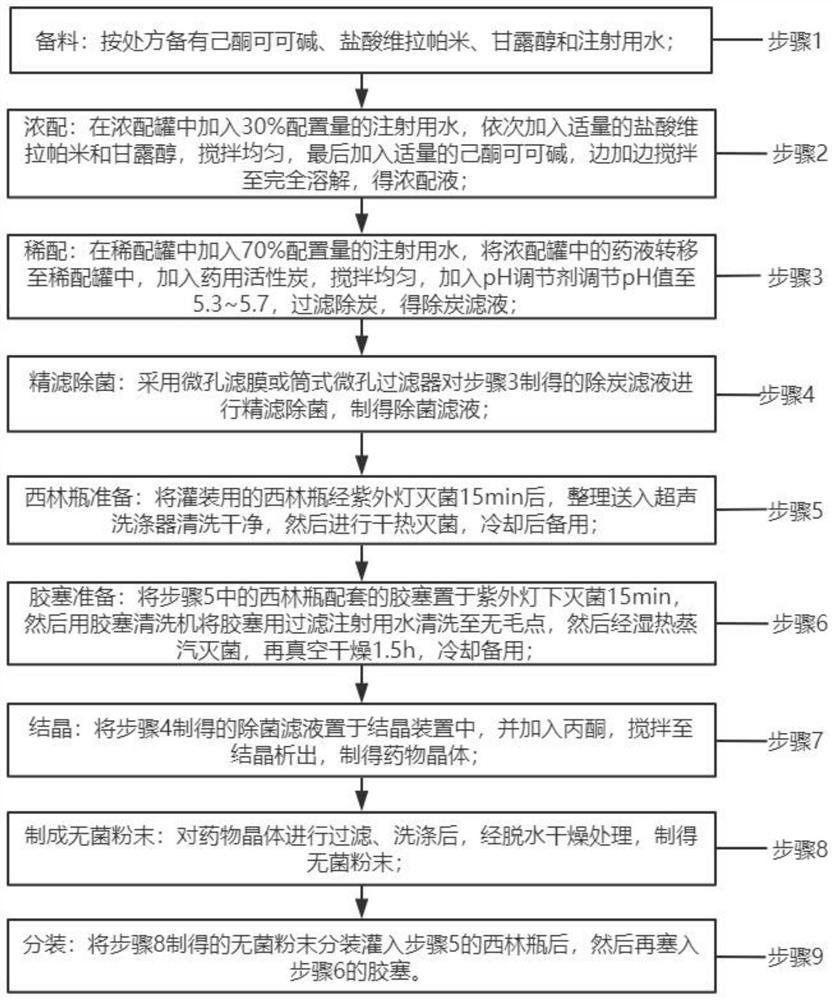
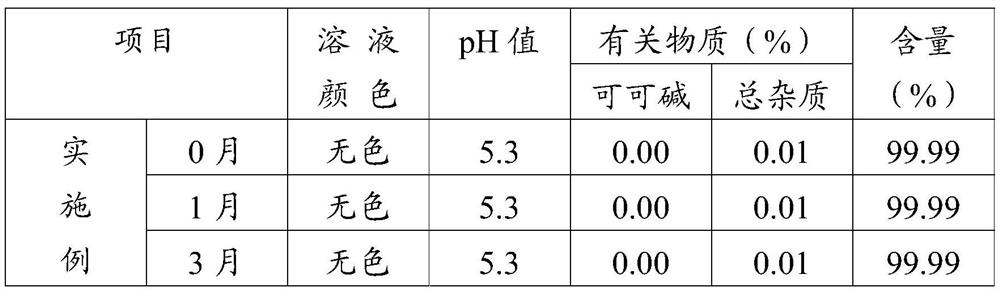
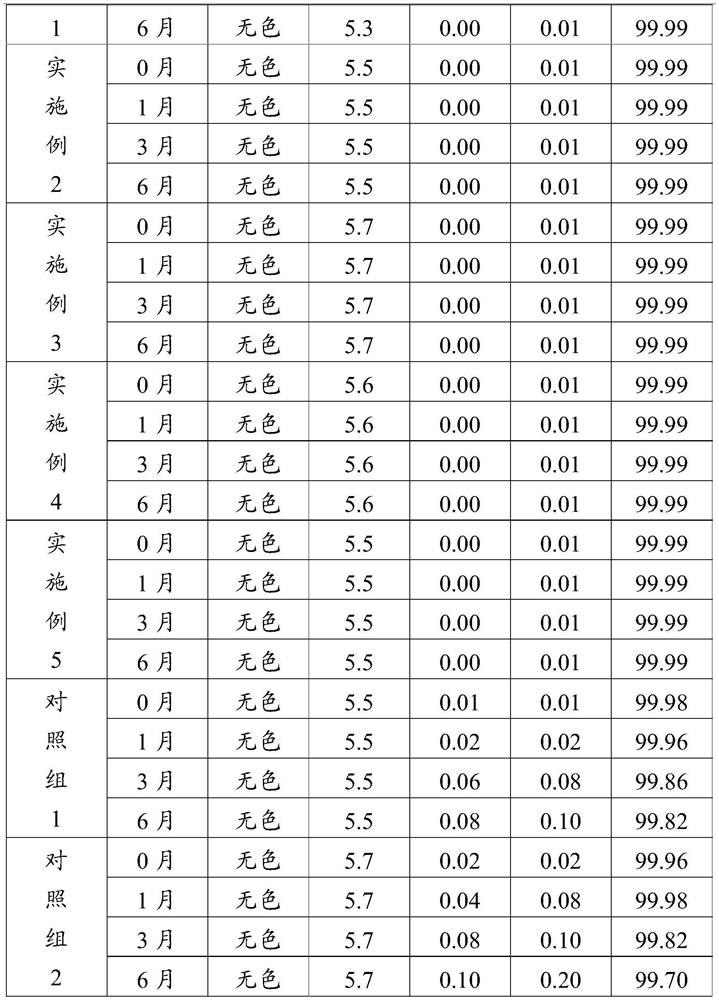
Preparation method of pentoxifylline powder for injection
InactiveCN113332291AImprove stabilityWell mixedPowder deliveryHydroxy compound active ingredientsVerapamil HydrochloridePenicillin
The invention discloses a preparation method of pentoxifylline powder for injection. The preparation method comprises nine steps of material preparation, concentrating, dilution, fine filtration and sterilization, preparation of penicillin bottles, preparation of rubber stoppers, crystallization, preparation of sterile powder and subpackaging. The preparation procedures are simple and easy in operations. Pentoxifylline, verapamil hydrochloride, mannitol and water for injection are sequentially concentrated and diluted in formula dosage, thereby ensuring thorough mixing of pentoxifylline, verapamil hydrochloride, mannitol and water for injection, and enhancing stability of the medicine. The invention provides the preparation method of the pentoxifylline powder for injection, and solves the technical problems of difficult preservation and easy generation of impurities at high temperature or under the light for existing pentoxifylline, thereby affecting curative effect.
Owner:HAINAN GENERAL & KANGLI PHARMA
Verapamil hydrochloride delayed-release capsule and preparation method thereof
ActiveCN101422453BSmall inter-individual variabilityReduce or eliminate irritationNitrile/isonitrile active ingredientsHeterocyclic compound active ingredientsVerapamil hclControlled release
Owner:LEPU PHARMACEUTICAL CO LTD
Jatrophane type diterpene derivative as well as preparation method and application thereof
ActiveCN113402483AHigh yieldEasy to operatePreparation from carboxylic acid halidesOrganic compound preparationPharmaceutical SubstancesMulti drug resistant
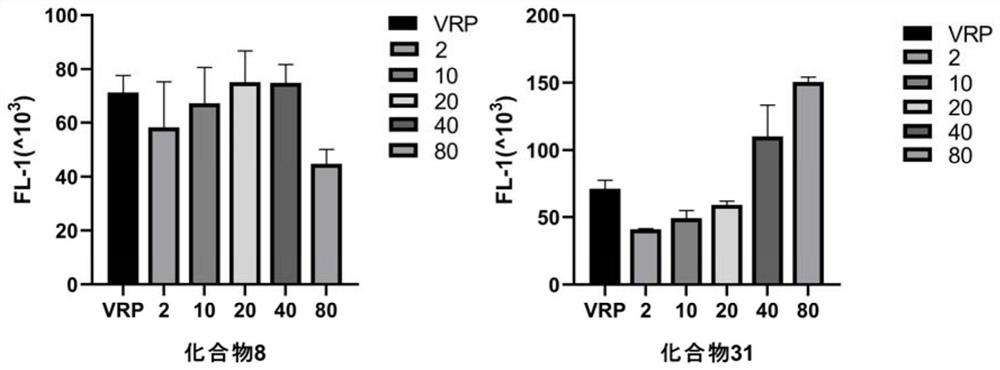
The invention relates to a jatrophane type diterpene derivative as well as a preparation method and application thereof. According to the derivative, a characteristic active component ES3 contained in euphorbia sororia fruits is used as a raw material, and several derivatization methods are adopted to obtain compounds 1-17; the reaction conditions are mild, the experimental steps are simple, and the synthesized compounds 1-17 are subjected to a preliminary multidrug resistance reversal activity test; and experimental results show that the compounds 1-17 show different degrees of multidrug resistance reversal activity, the drug resistance of drug-resistant cells to antitumor drugs can be reversed to different degrees when the compounds 1-17 are combined with the antitumor drugs, the compounds 1, 2, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16 or 17 have high multidrug resistance reversal activity, and the reversal multiple thereof is higher than that of a positive control drug verapamil, wherein the reversal multiple of the compound 12 is the highest. The kind of compounds can be used as a tumor multidrug resistance reversal drug.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Application of hematoporphyrin derivative in treatment of bladder cancer together with chemical drug
PendingCN112546235AGood reversal effectEnergy modified materialsInorganic active ingredientsOncologyChemo therapy
The invention belongs to the field of chemotherapeutic drug sensitivity enhancing medicine and particularly relates to use of a hematoporphyrin-verapamil-fragment-combined hematoporphyrin derivative A2 in tolerance and sensitivity enhancing of bladder cancer reversing chemotherapeutic drugs. The hematoporphyrin derivative A2 has different extents of chemotherapeutic-drug-sensitivity-enhanced bladder cancer cell proliferation inhibiting actions, and it is shown that the hematoporphyrin derivative A2 can be developed into chemotherapeutic-drug sensitizing agents or drug resistance reversing agents and is applied to treatment on bladder cancer sufferers.
Owner:范平生 +5
Construction method and application of p-glycoprotein model of human small intestine 3D organoid research
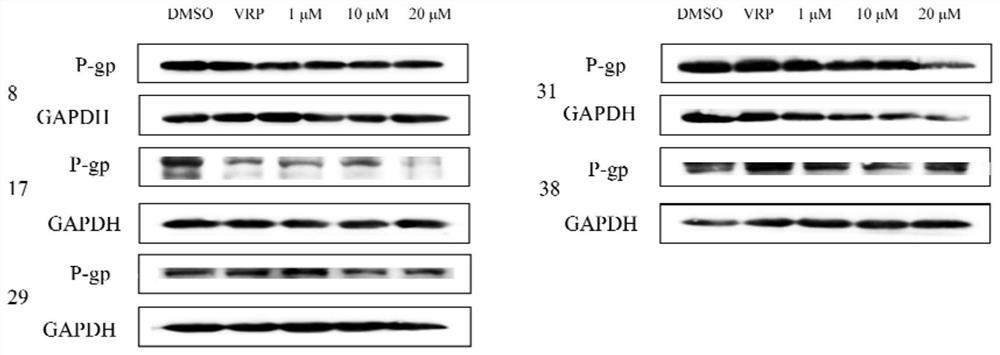
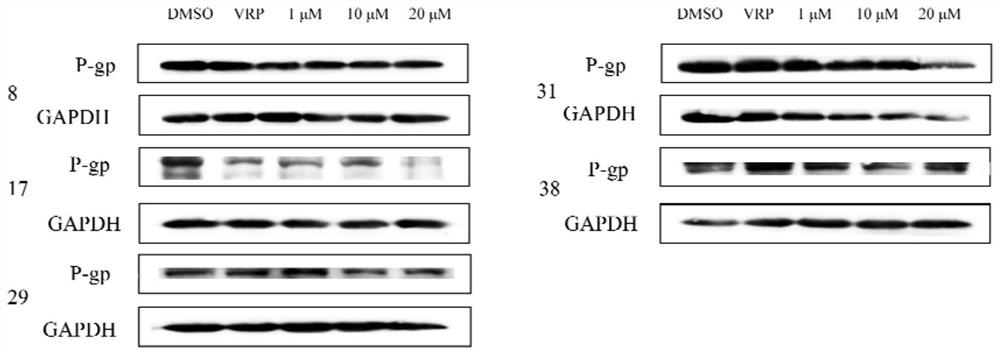
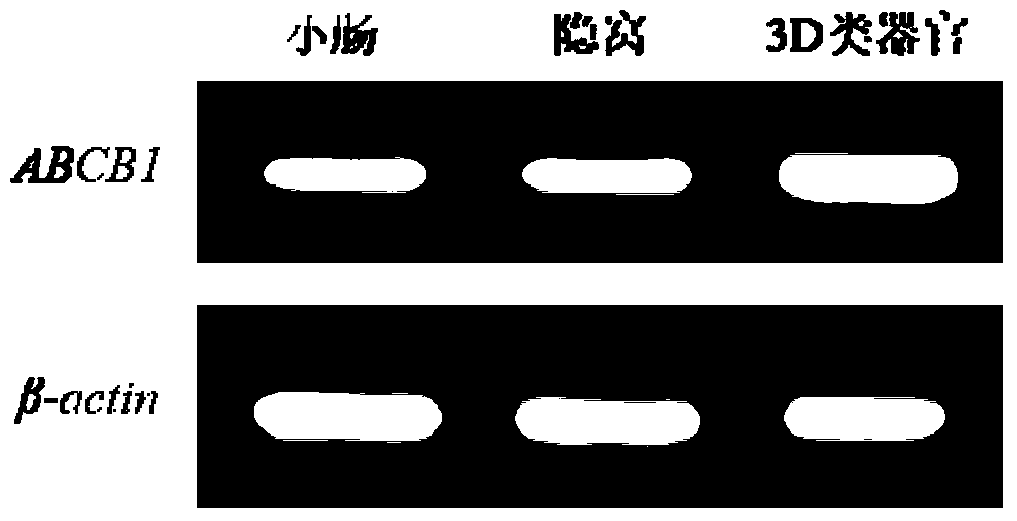
ActiveCN105950539BShort training periodSave time and costGastrointestinal cellsMicrobiological testing/measurementBiotechnologyMatrigel
The invention discloses a method for building P-glycoprotein (P-gp) research models based on human small intestine 3D (three-dimensional) organoid. The method includes inoculating human small intestine crypts in matrigel at first, adding ADMEM / F12 culture media with specific growth factors into the matrigel and cultivating the human small intestine crypts to form 3D organoids; carrying out morphological observation and detecting expression of P-gp from mRNA [messenger RNA (ribonucleic acid)] and protein level; researching influence of Verapamil and Mitotane on Rh123 transportation by the aid of a co-incubation process by Rhodamine 123 (Rh123) which is used as a substrate. The method has the advantages that the P-glycoprotein research models based on human small intestine 3D organoid can be applied to P-gp-mediated medicine transport research and also can be widely applied to in-vitro high-throughput screening on P-gp inhibitors, and the method is high in efficiency and speed.
Owner:EAST CHINA NORMAL UNIV
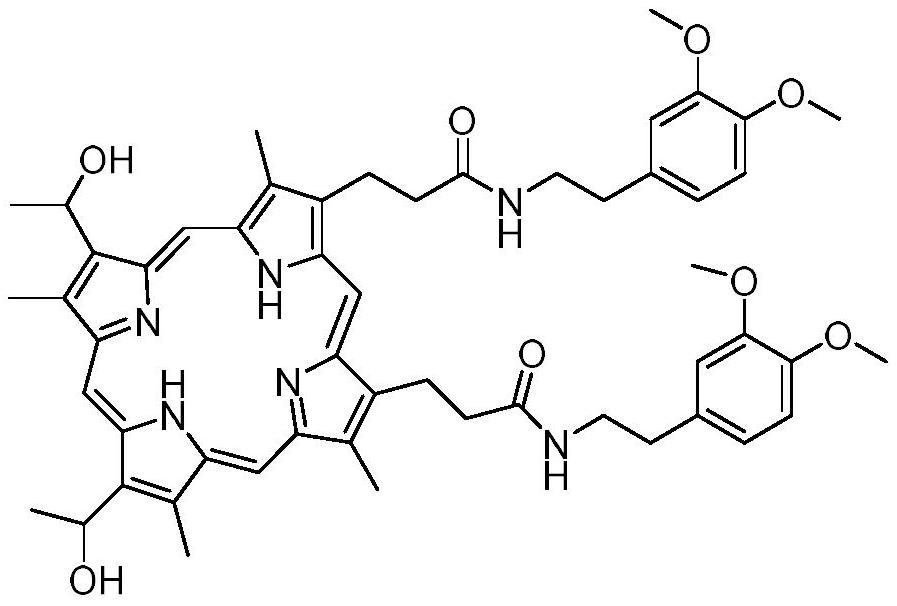
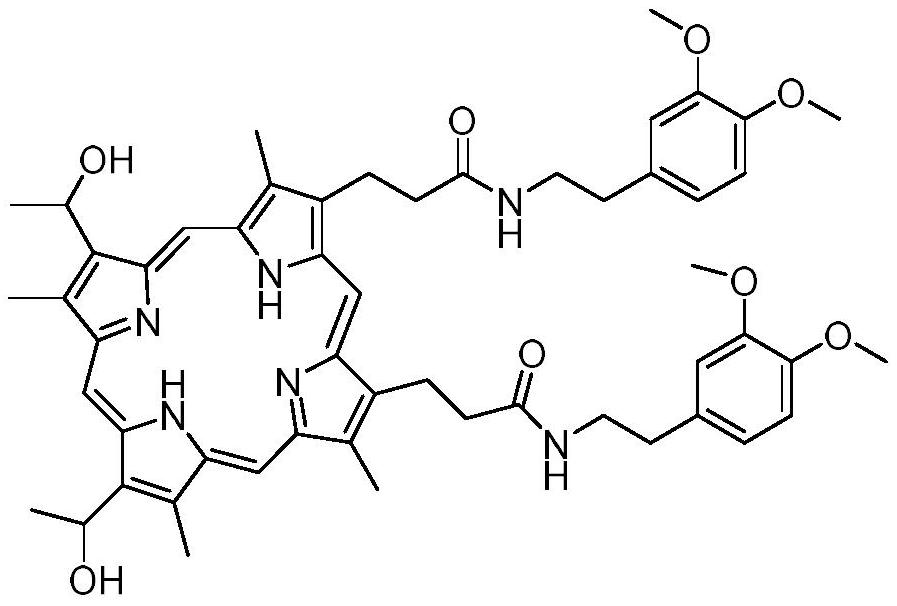
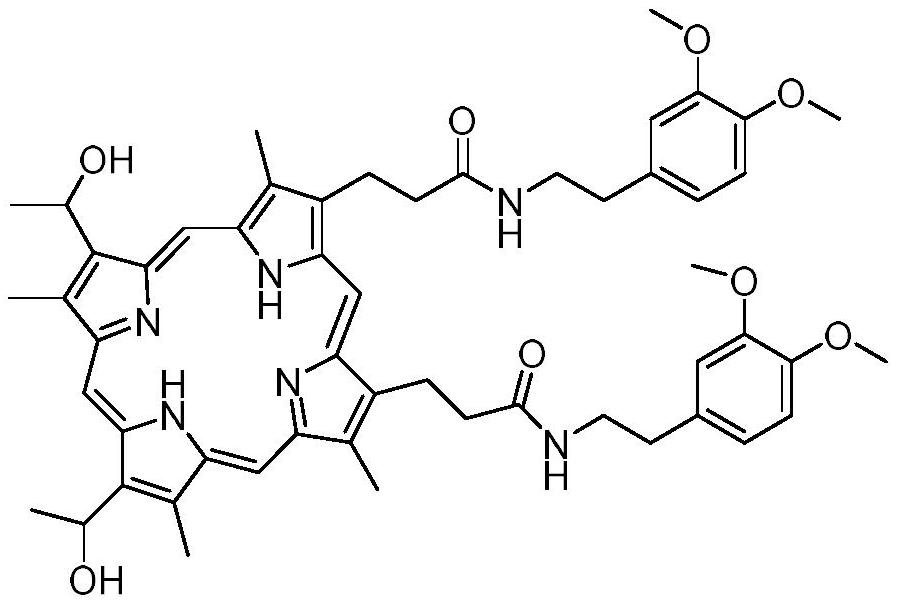
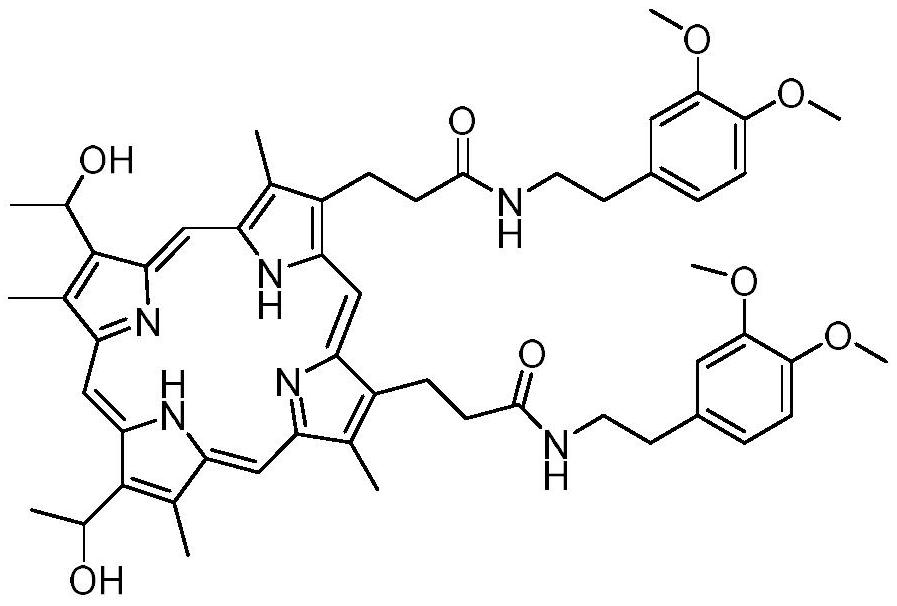
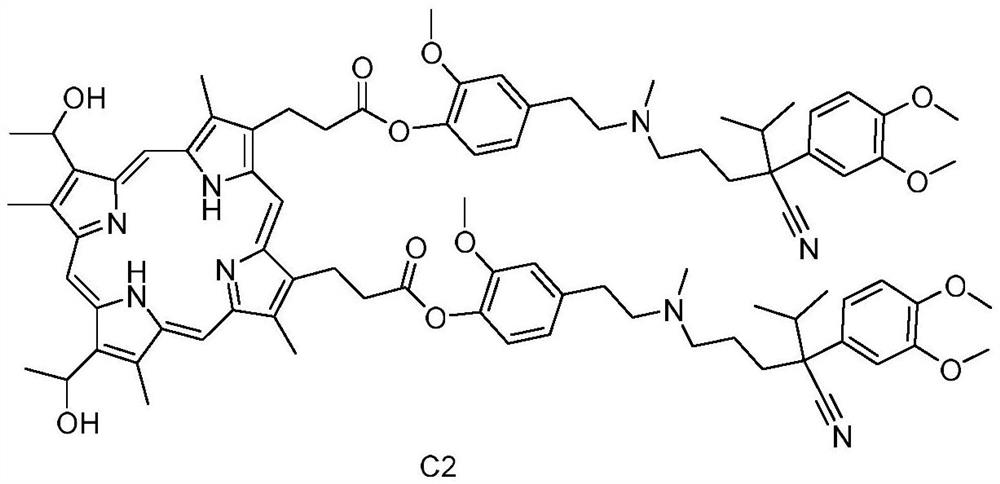
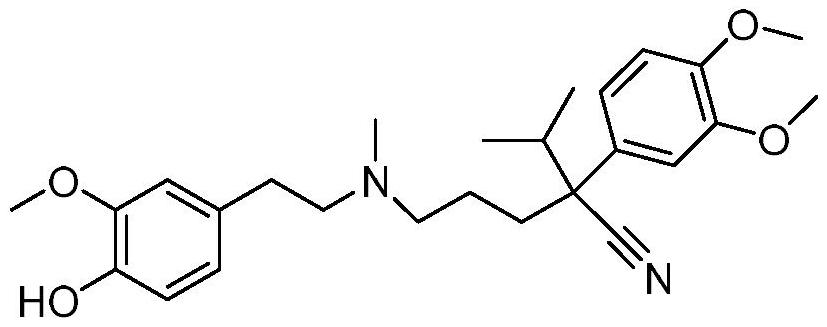
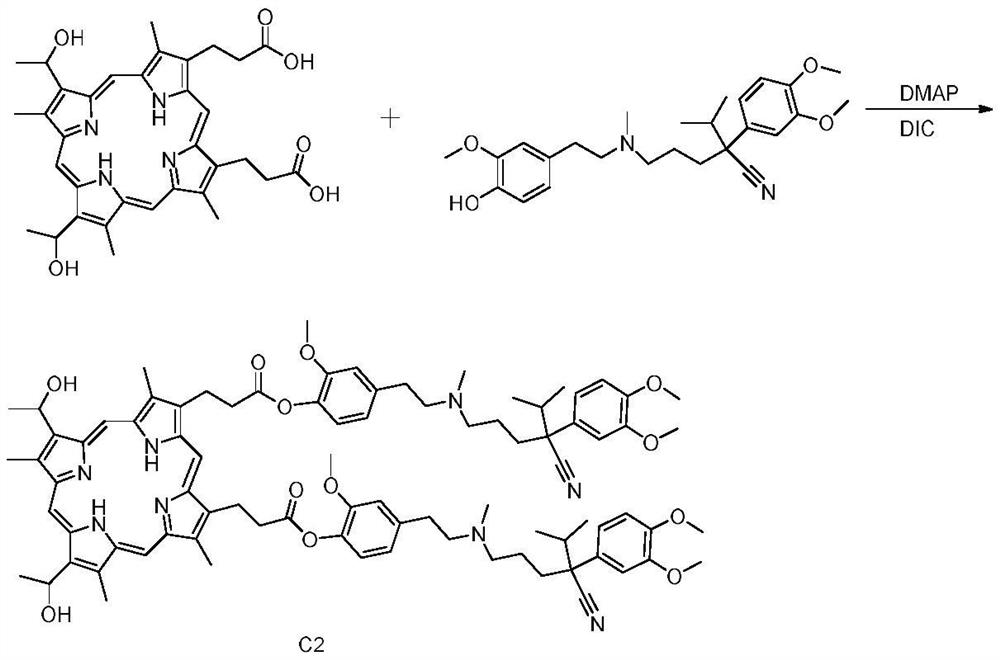
Preparation method and application of hemoporphyrin/verapamil conjugates
InactiveCN112707911AImprove physical and chemical propertiesOrganic chemistryEnergy modified materialsPharmaceutical drugMedicinal chemistry
The invention relates to the field of medicinal chemistry, in particular to preparation of hemoporphyrin / verapamil conjugates C1 and C2 and application of the hemoporphyrin / verapamil conjugates in preparation of anti-tumor drugs. Researches prove that the hemoporphyrin / verapamil conjugates disclosed by the invention can be used for remarkably inhibiting the activity of various tumor cells and achieving the treatment and control effects of resistant tumors.
Owner:范平生 +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com