Moleplant seed diterpene derivatives and application thereof
A technology of stephenia and derivatives, which is applied in the field of stephenia diterpene derivatives, and can solve problems such as insignificant reversal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Preparation and determination of compounds 1-41
[0079] 1. Preparation of compounds 1, 37 and 40. In a 1% (m / v) NaOH / MeOH solution system, compounds 18, 38, and 39 were respectively used as raw materials (compound 18 corresponds to compound 1, and so on), and reacted at room temperature for 24 h. After the reaction, it was extracted with ethyl acetate, concentrated, and separated by chromatographic column (eluted with a mixed solution of petroleum ether and ethyl acetate) to obtain the target product. The NMR data of compounds 1, 37, and 40 are shown below, respectively.
[0080] Compound 1: HRMS-ESI-TOF: [M+Na]+373; mp 226-228°C; 1 H NMR (400MHz, Acetone) δ7.29 (d, J = 11.6Hz, 1H, H-12), 5.07 (s, 1H, H-17α), 4.96 (d, J = 8.5Hz, 1H, H-7 ),4.81(s,1H,17β),4.68(s,1H,OH-15),4.44(s,1H,H-5),4.37(s,1H,H-3),4.32(s,2H, OH-5,OH-7),4.06(s,1H,OH-3),3.07(dd,J=13.2,8.8Hz,1H,H-1α),2.21(dd,J=8.4,3.3Hz,1H ,H-4),1.96–1.85(m,2H,H-2,H-8α),1.69–1.61(m,4H,H-1β,H-20),1.45(dd,...
Embodiment 2
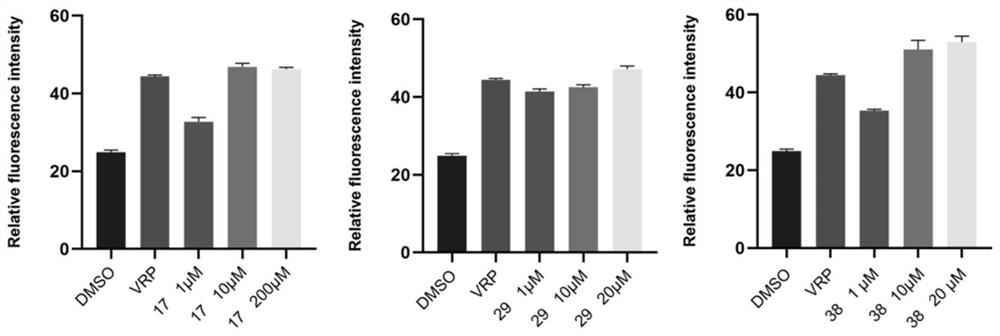
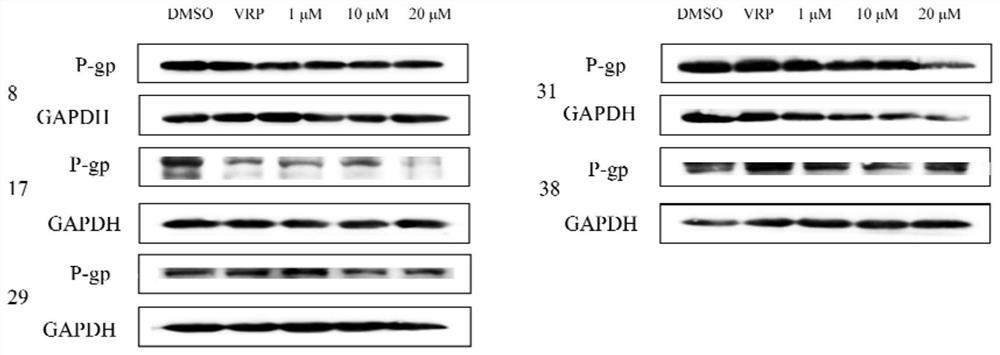
[0135] Embodiment 2: In vitro activity test of compounds 1-41
[0136] 1. Cytotoxicity detection of diterpene derivatives of stephenae. Specific operation: Inoculate MCF-7 and MCF-7 / ADR cells in a 96-well plate according to the limited number of 1×110 cells / well, add 100 μL of MEM medium containing 10% fetal bovine serum to each well and culture for 15 hours until the cells grow To cover 70% of the well area, compound No. 1-41 with a final concentration gradient of 1 μM, 5 μM, 20 μM, 50 μM, and 100 μM was sequentially added. The compounds were all dissolved in DMSO, and the administration volume was 1 μL. Doxorubicin with a final concentration gradient of 0.01 μM, 0.1 μM, 1 μM, 5 μM, and 10 μM was set as a positive control, the compound was dissolved in DMSO, and the administration volume was 1 μL. Set DMSO as the negative control, and the administration volume was 1 μL. Three wells were set up in each group as parallel samples. After culturing at 37°C for 48 hours, the ori...
Embodiment 3
[0148] Example 3: Intracellular Aggregation Experiment
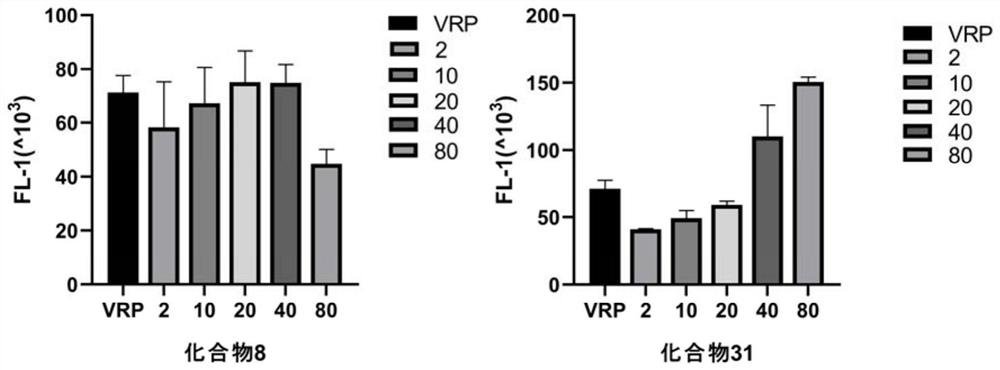
[0149] This example provides the intracellular aggregation test of compounds 8 and 31 for Rhodamine 123 (Rh123). Specific operation: MCF-7 / ADR and MCF-7 cells were mixed with 2×10 6 Inoculate into 24-well plates at a density of 24 wells, incubate at 37°C for 16 h, pre-treat with compound 8 and compound 31 (concentrations of 2, 10 and 20 μM, respectively) screened for drug resistance reversal for 1 h, and use a final concentration of 20 μM Verapamil was used as a positive control. The cells were then incubated with Rh123 at a final concentration of 5 μM at 37°C in the dark for 1 h. Cells were washed twice with PBS, resuspended in PBS buffer, and analyzed by flow cytometry. Experimental results such as figure 1 , Table 3. figure 1 It is a schematic diagram of the rhodamine accumulation changes of compound 8 and compound 31 in MCF-7 / ADR cells respectively.
[0150] Table 3 Compounds 8 and 31 inhibit P-gp-mediated rhodam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com