Preparation method of pentoxifylline powder for injection
A technology of pentoxifylline and theobromine powder, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, powder transportation, etc. Preservation and other issues, to achieve the effect of improving quality, complete and pure crystallization, and strengthening stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
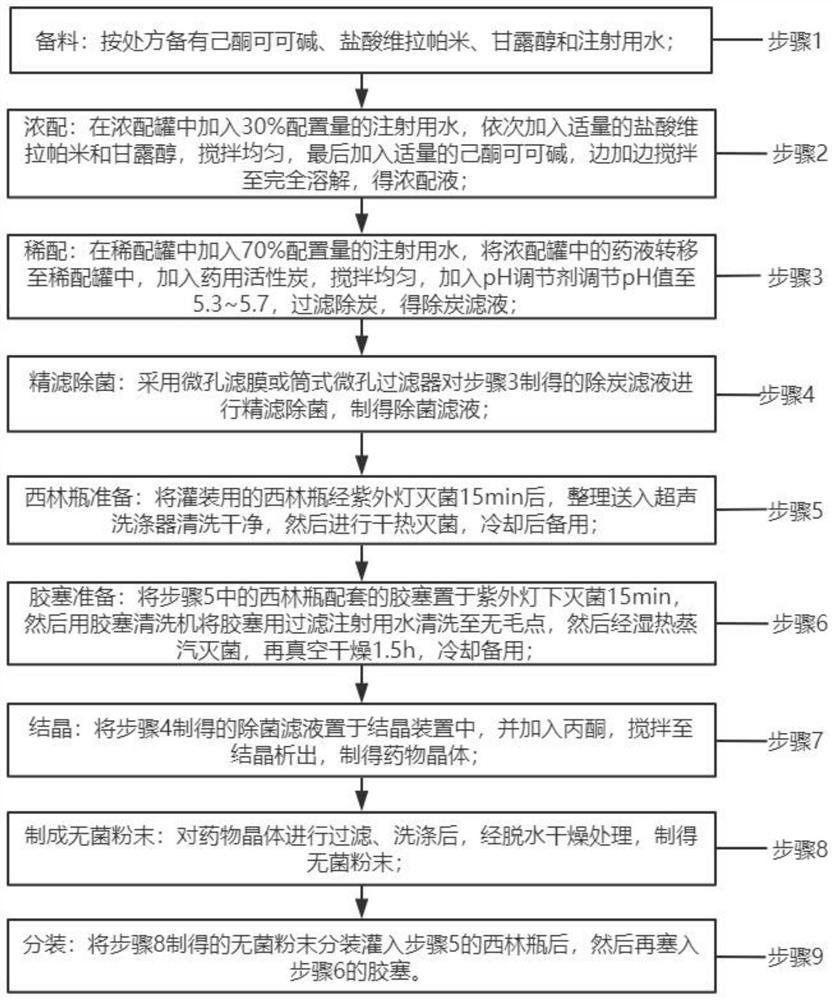
[0032] For ease of understanding, see figure 1 , an embodiment of the preparation method of the pentoxifylline powder for injection provided by the application comprises the following steps:
[0033] Step 1, material preparation: prepare pentoxifylline, verapamil hydrochloride, mannitol and water for injection according to the prescription;
[0034] Step 2. Concentrated preparation: add 30% water for injection in the concentrated preparation tank, add appropriate amount of verapamil hydrochloride and mannitol in turn, stir evenly, finally add appropriate amount of pentoxifylline, and stir until Dissolve completely, get concentrated solution;
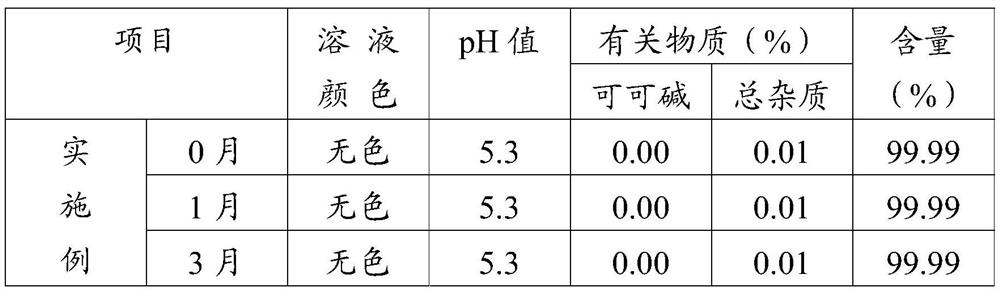
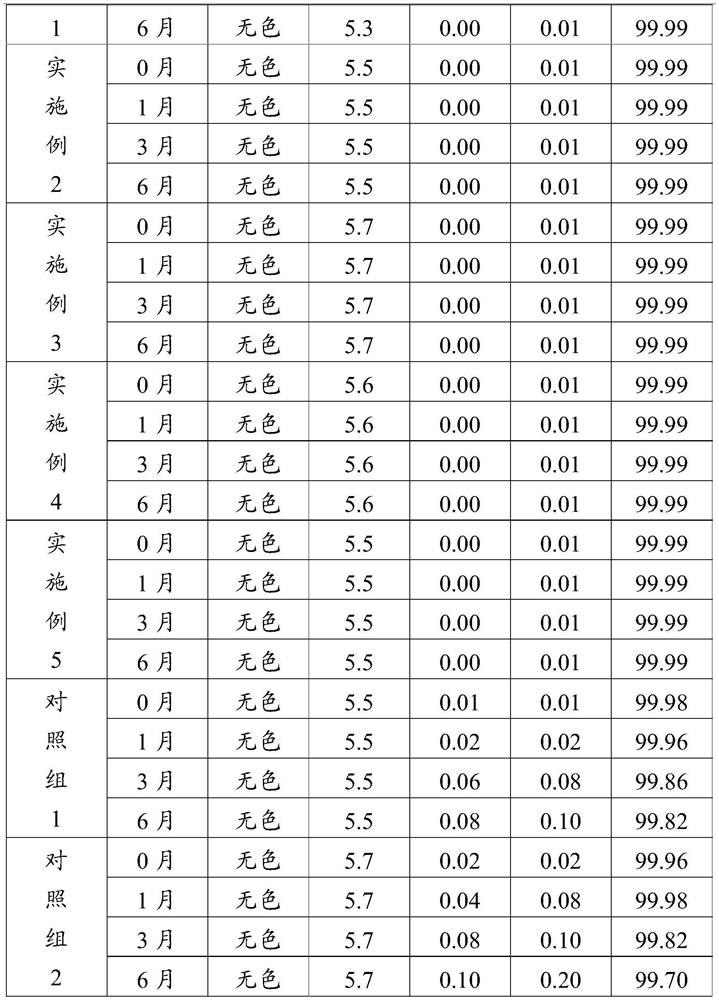
[0035] Step 3. Dilute formulation: add 70% of the configured water for injection into the dilute formulation tank, transfer the liquid medicine in the concentrated formulation tank to the dilute formulation tank, add medicinal activated carbon, stir evenly, add a pH regulator to adjust the pH value To 5.3~5.7, filter to remove charcoal...
Embodiment 2
[0044] Preparation method of pentoxifylline powder injection for injection
[0045] prescription:
[0046] 10 parts by weight of pentoxifylline
[0047] 2 parts by weight of verapamil hydrochloride
[0048] 10 parts by weight mannitol
[0049] 200 parts by weight of water for injection
[0050] Preparation Process:
[0051] Add 60 parts by weight of water for injection into the concentrated preparation tank, then add 10 parts by weight of pentoxifylline, 2 parts by weight of verapamil hydrochloride and 10 parts by weight of mannitol, and stir evenly to obtain a concentrated solution; Add 140 parts by weight of water for injection into the tank, keep the temperature of the water for injection at 75°C, transfer the concentrated solution to the dilute tank, add medicinal activated carbon to stir, add fumaric acid to adjust the pH value to 5.5, filter to remove charcoal to obtain the carbon-removing filtrate; use 0.45 μm and 0.22 μm microporous membranes in turn to carry out ...
Embodiment 3
[0053] Preparation method of pentoxifylline powder injection for injection
[0054] prescription:
[0055] 12 parts by weight of pentoxifylline
[0056] 2 parts by weight of verapamil hydrochloride
[0057] 15 parts by weight mannitol
[0058] 300 parts by weight of water for injection
[0059] Preparation Process:
[0060] Add 70 parts by weight of water for injection in the concentrated preparation tank, then add 12 parts by weight of pentoxifylline, 2 parts by weight of verapamil hydrochloride and 15 parts by weight of mannitol, and stir evenly to obtain a concentrated solution; Add 230 parts by weight of water for injection into the tank, keep the temperature of the water for injection at 85°C, transfer the concentrated solution to the dilute tank, add medicinal activated carbon for stirring, add fumaric acid to adjust the pH value to 5.7, filter to remove charcoal to obtain the carbon-removing filtrate; use 0.45 μm and 0.22 μm microporous membranes in turn to carry o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com