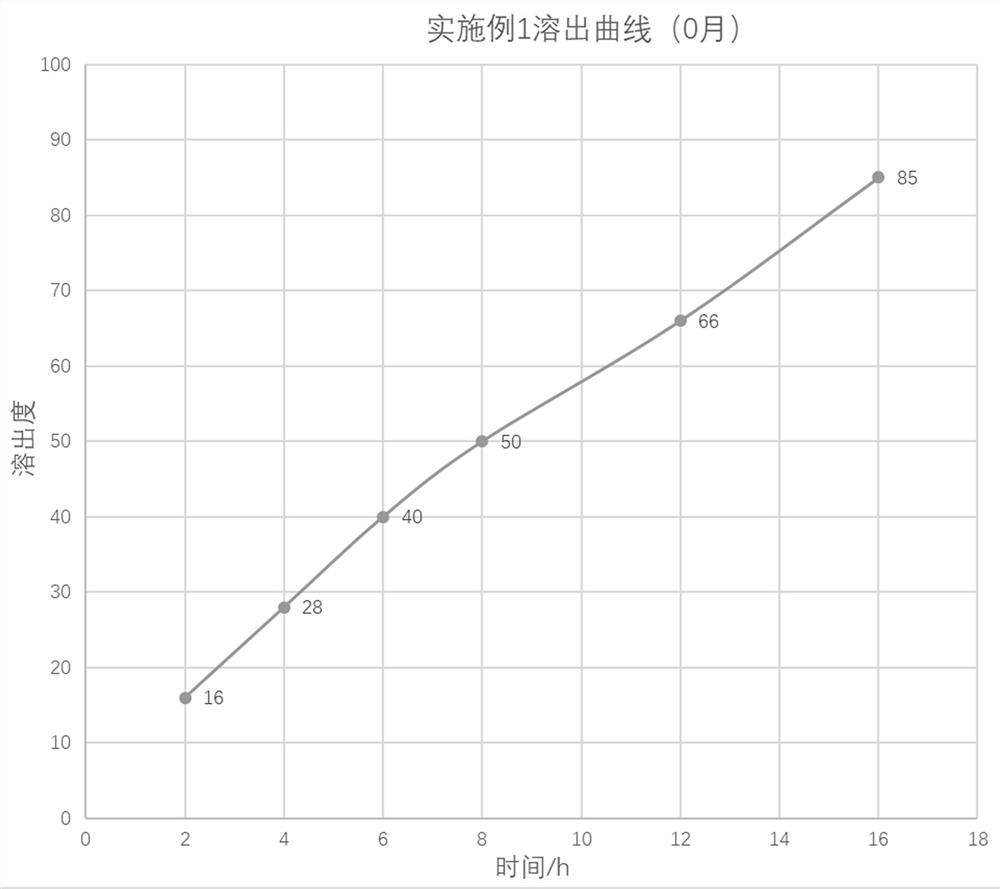
Patents
Literature
40 results about "Pentoxyfylline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
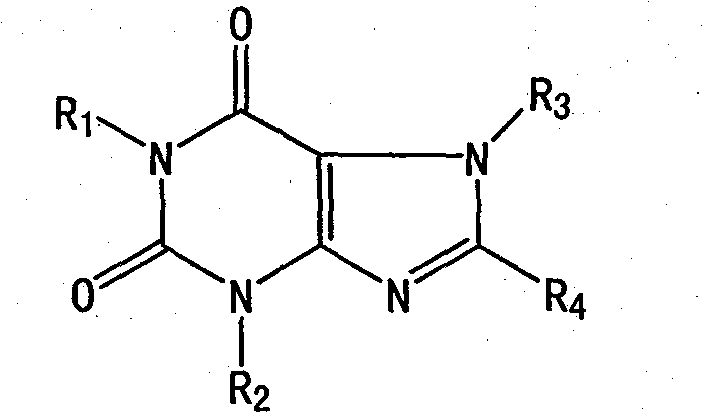
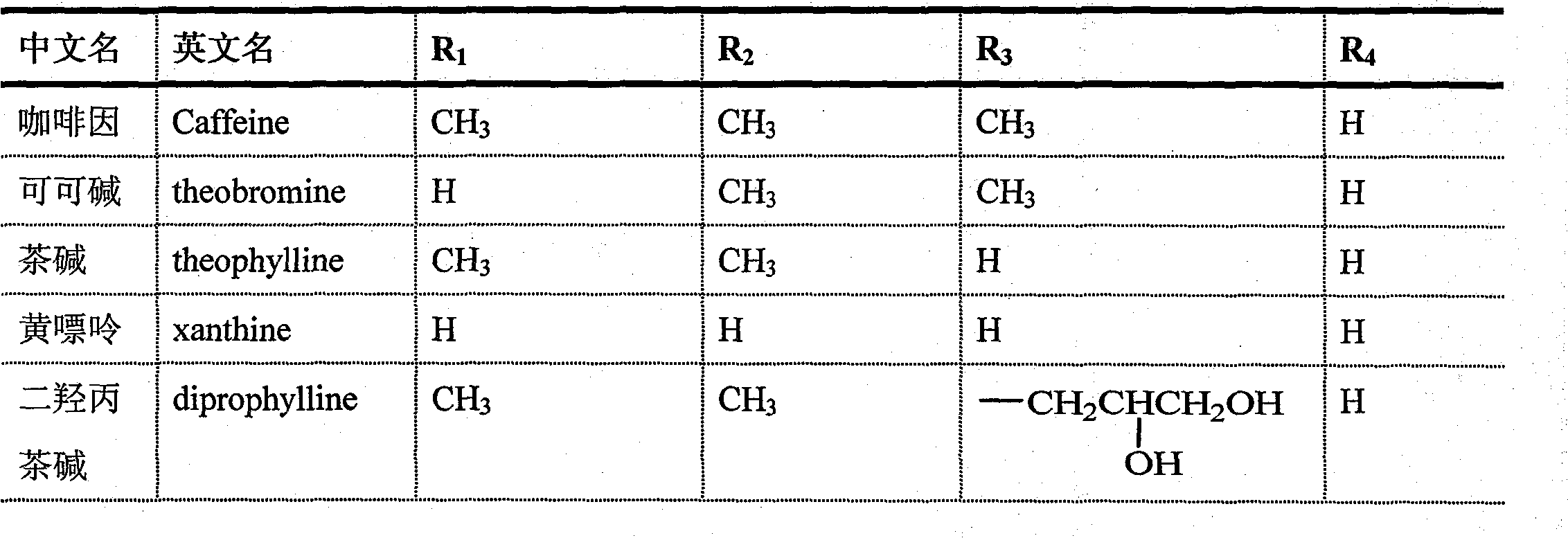
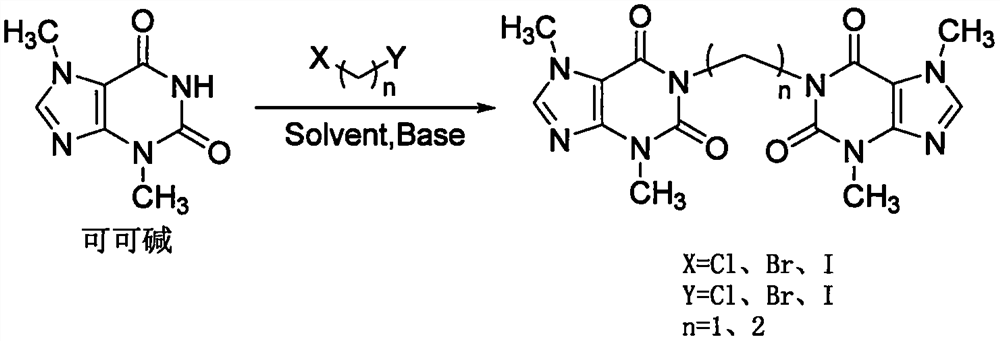
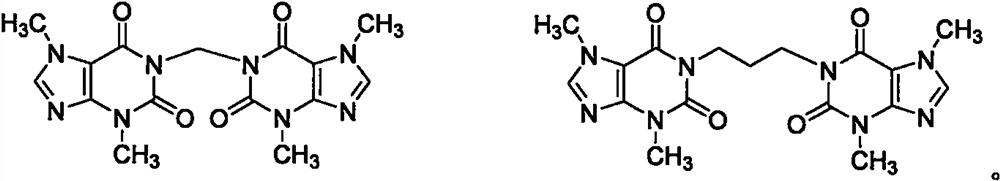
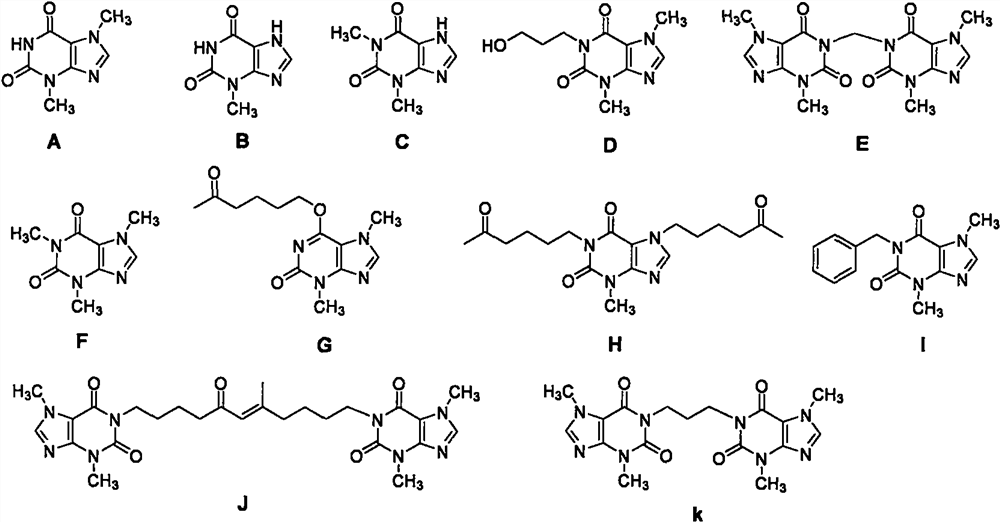
Substituted xanthine derivatives
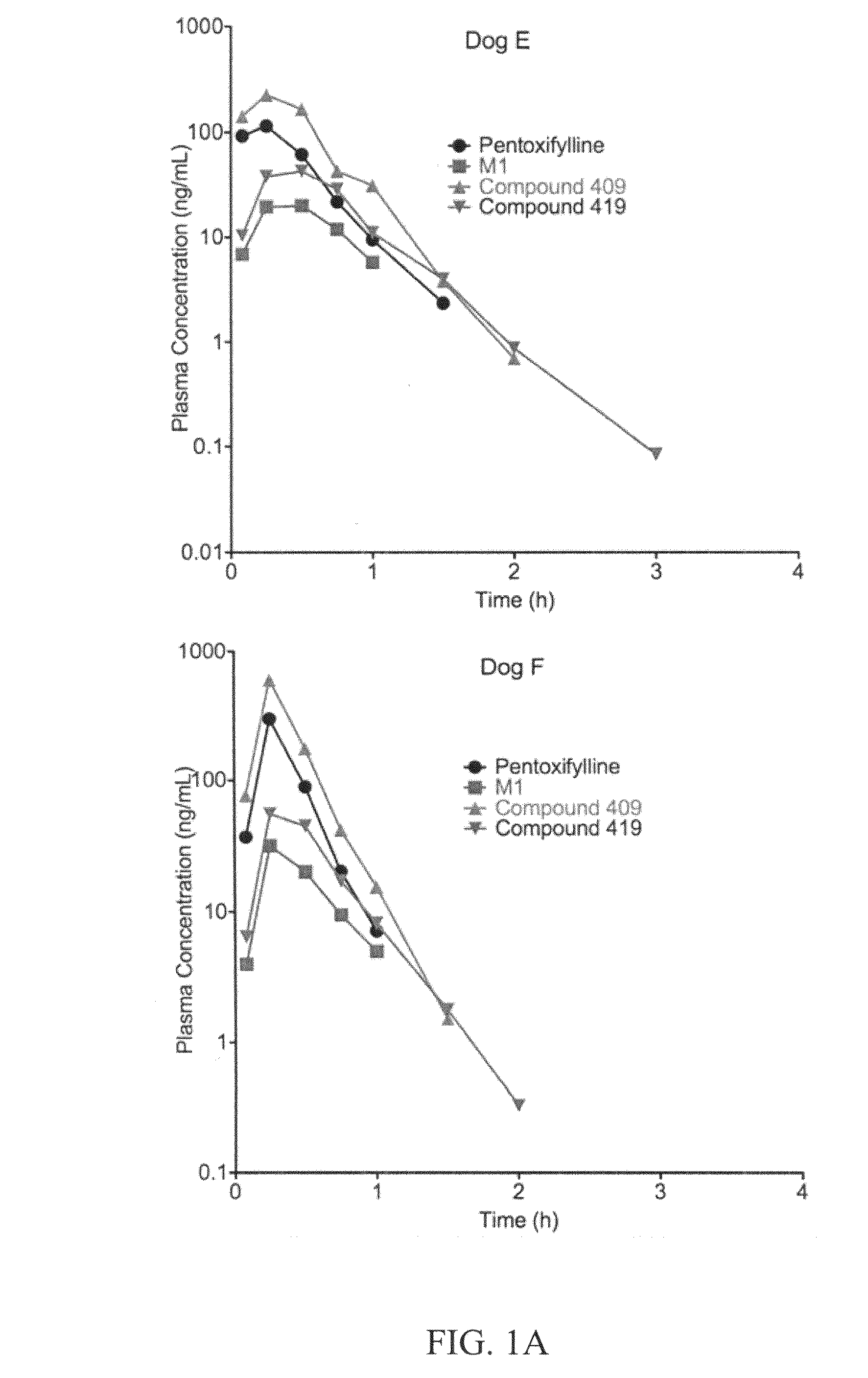
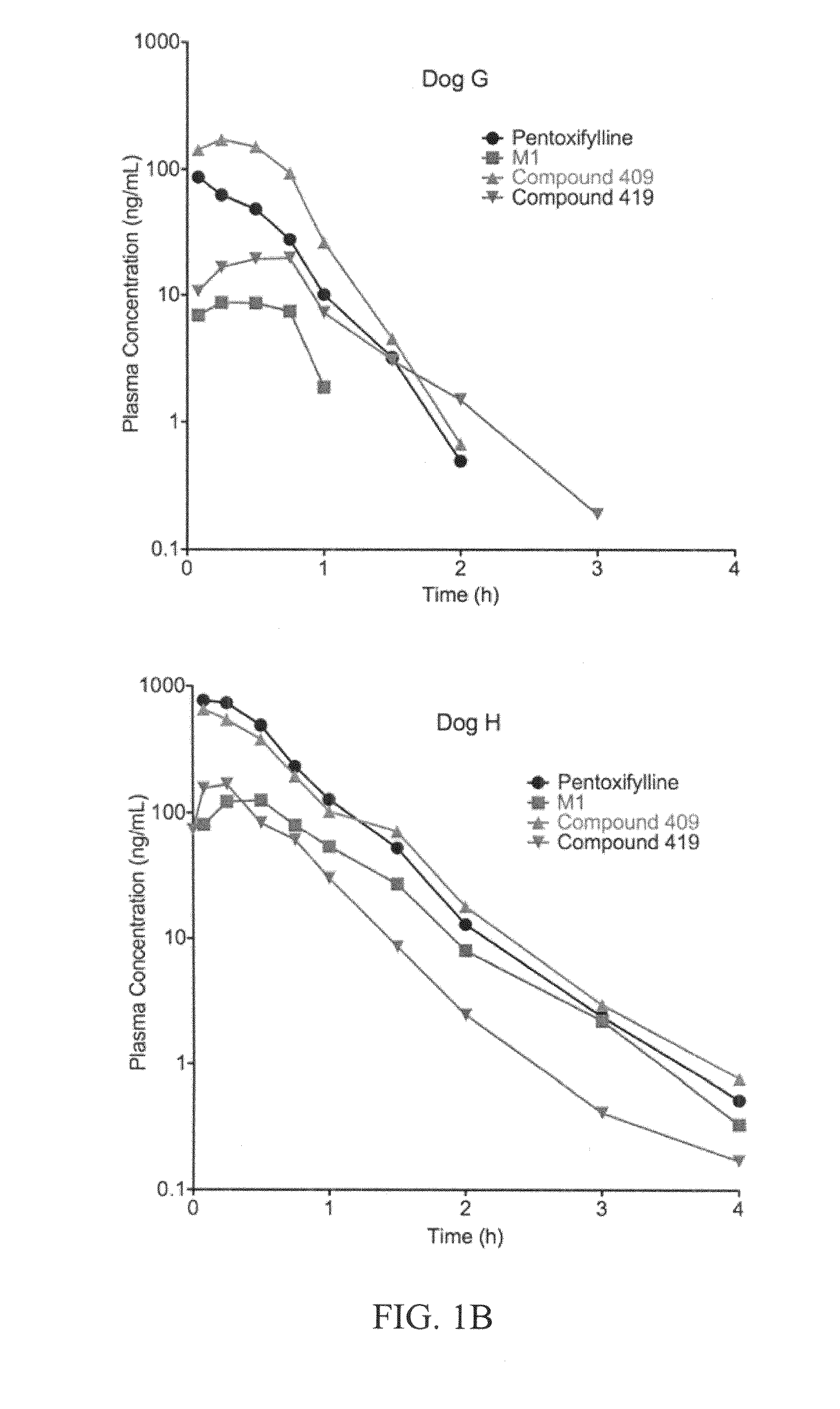
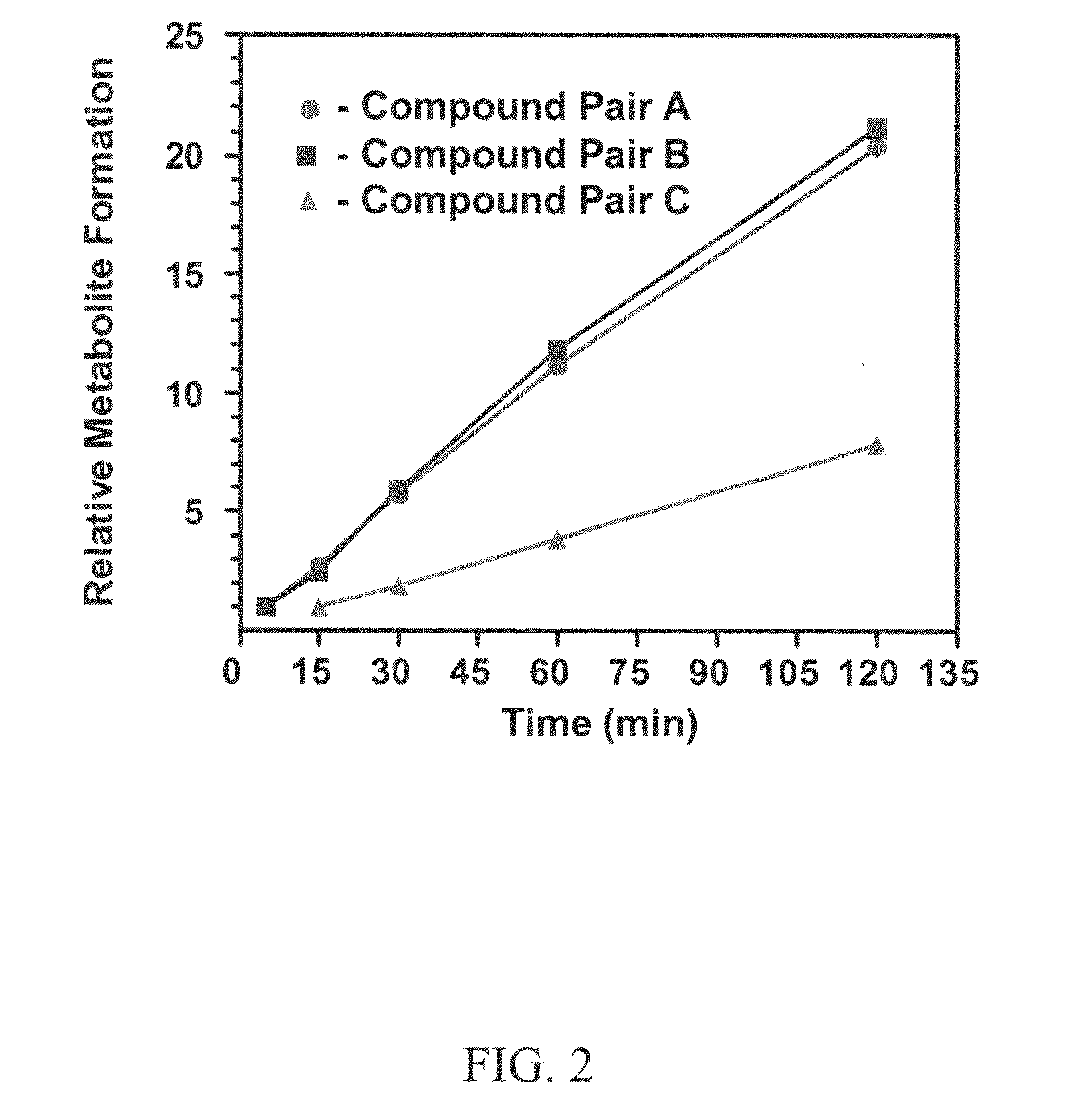
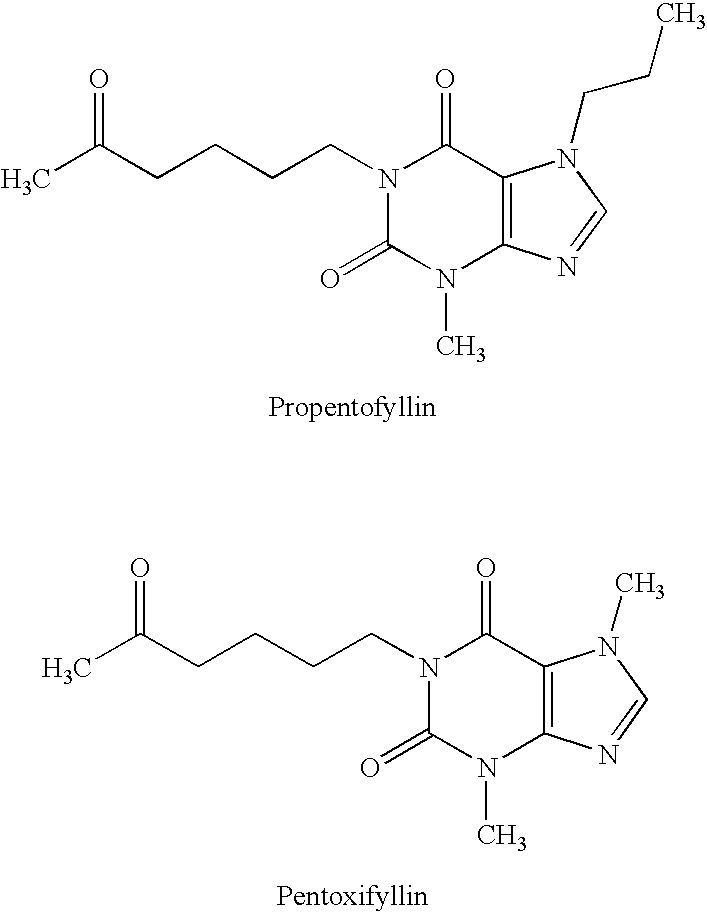
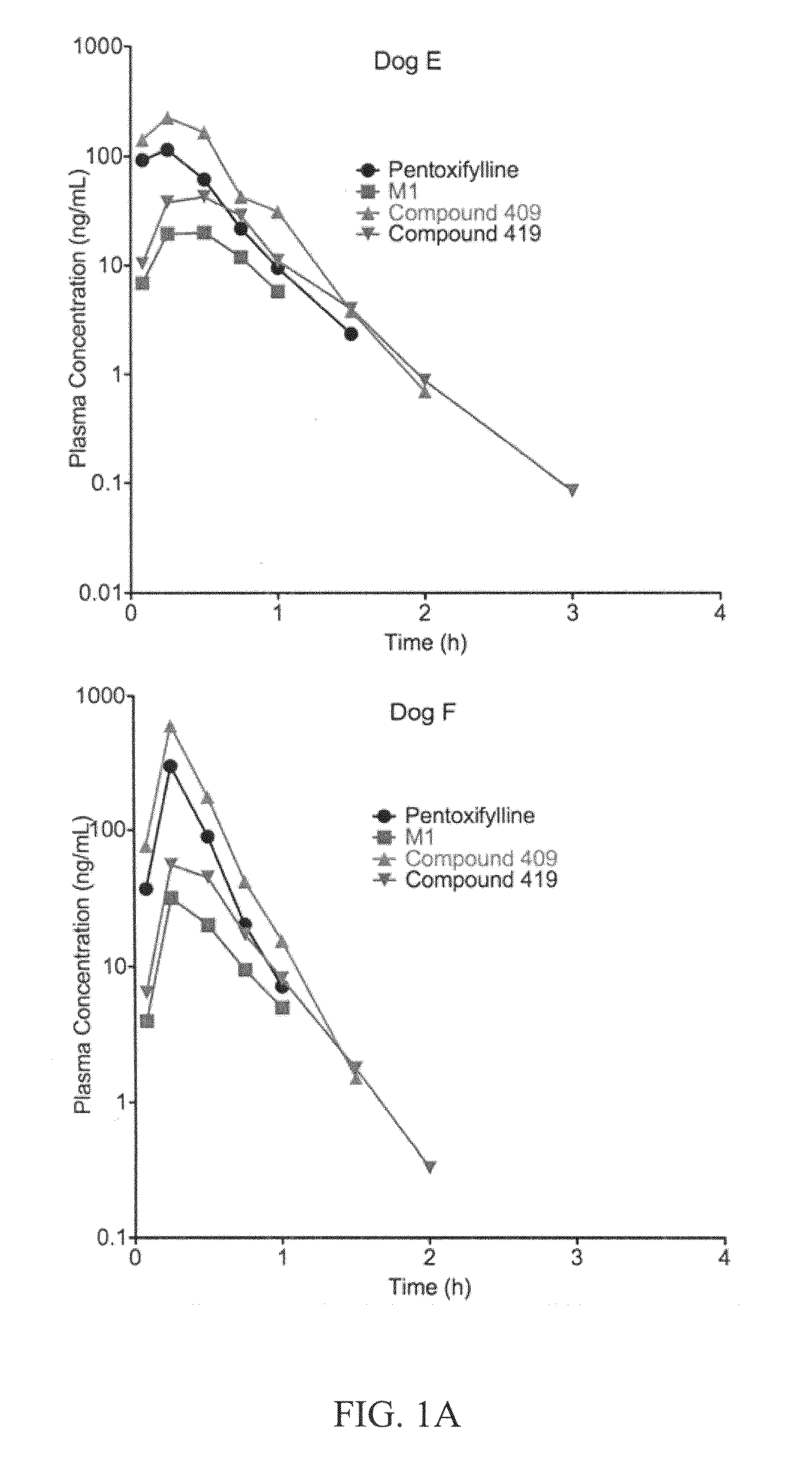
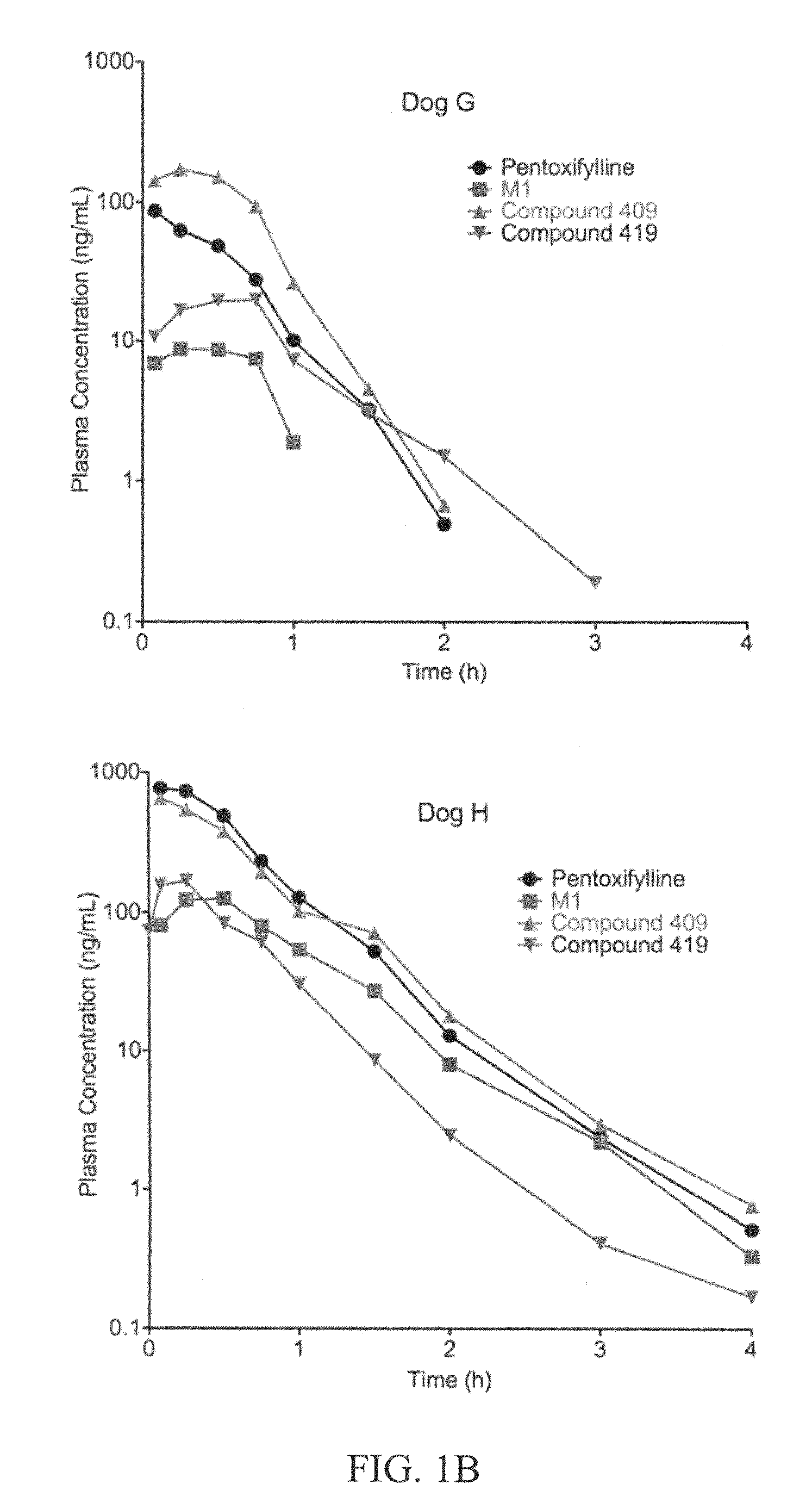
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
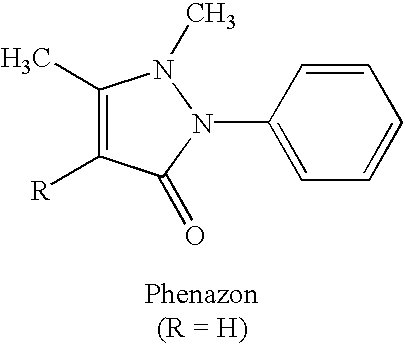
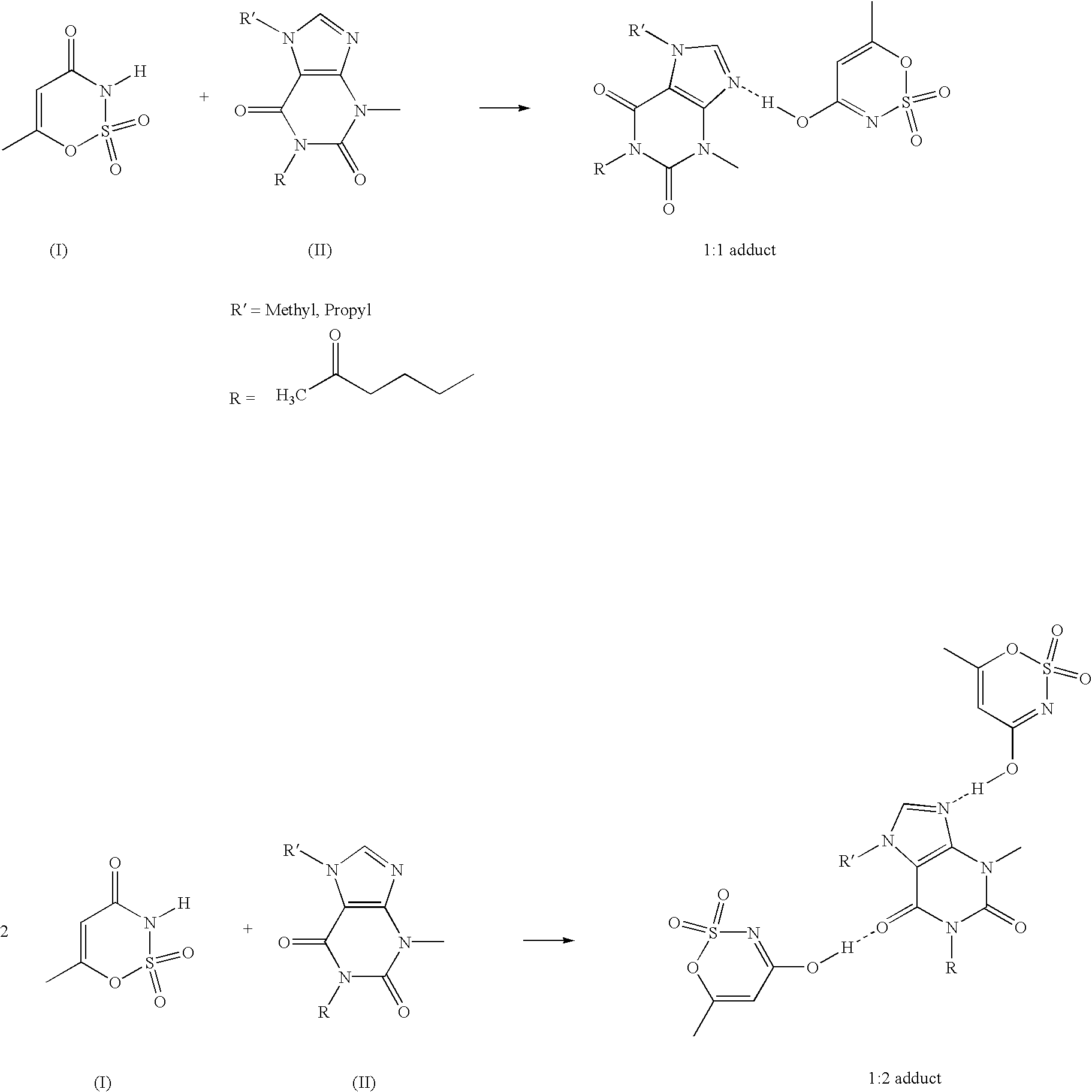
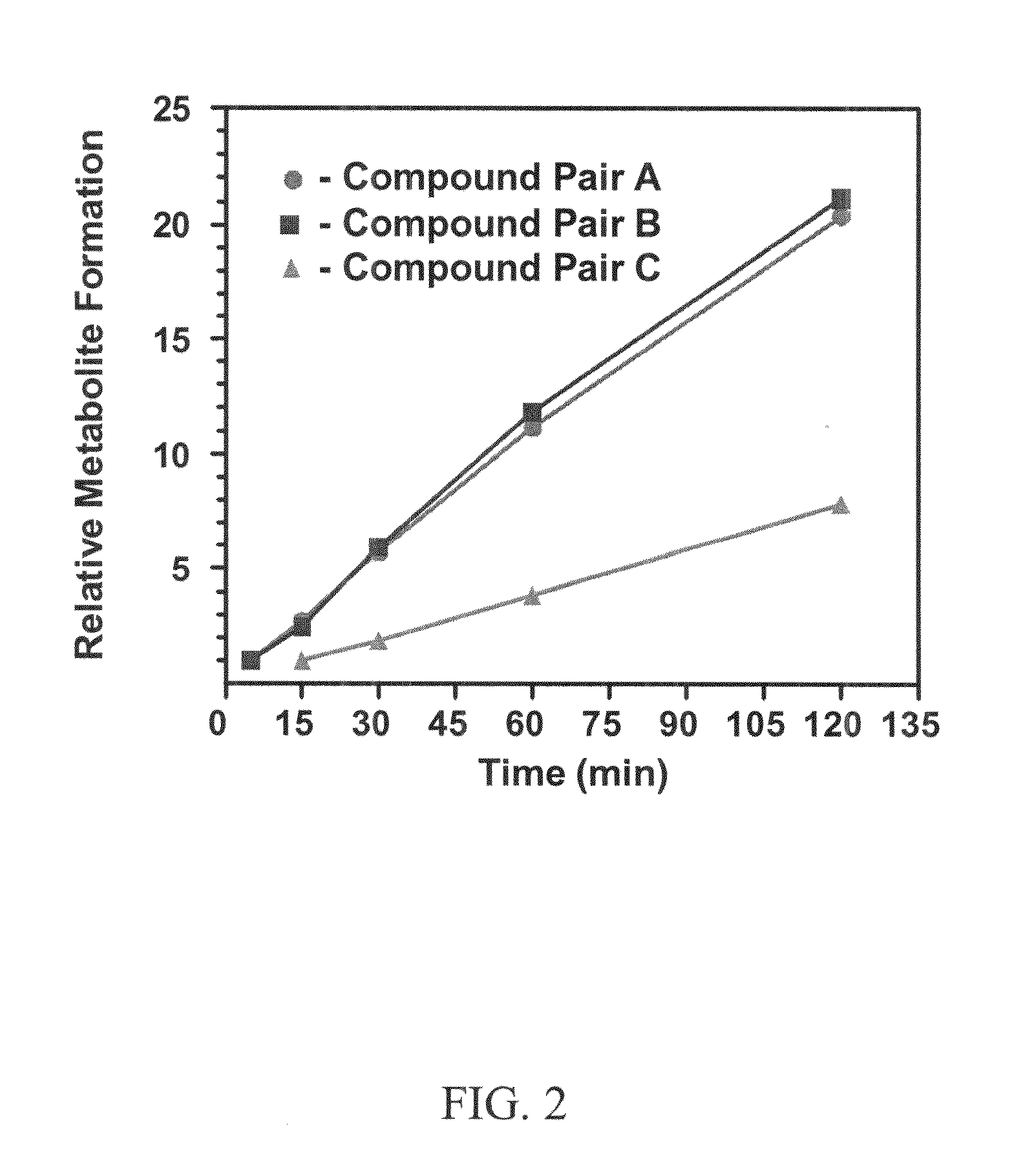
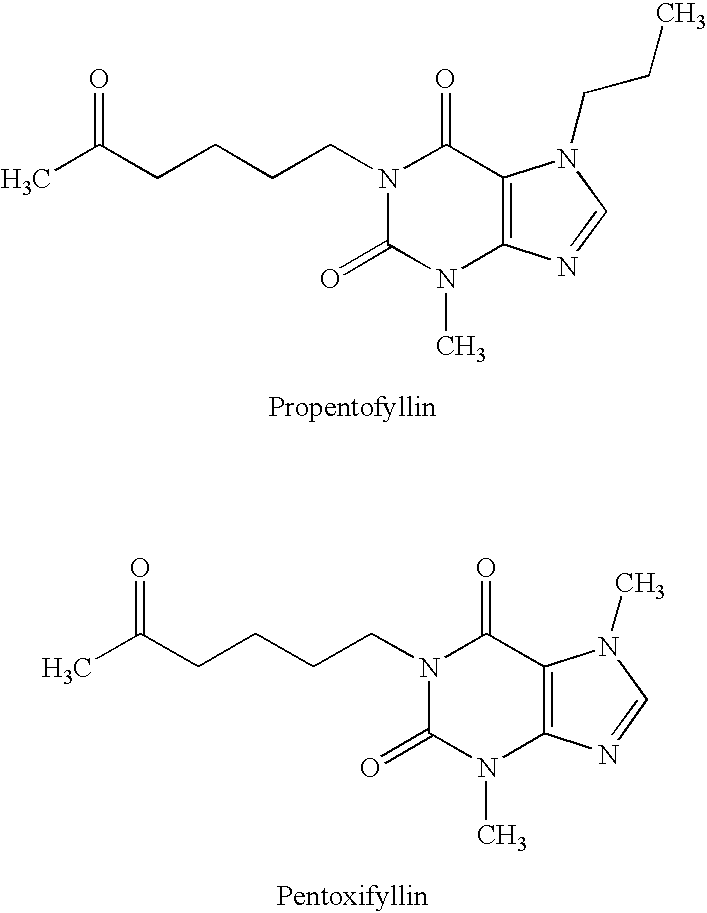
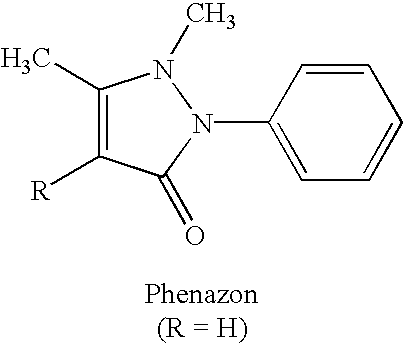
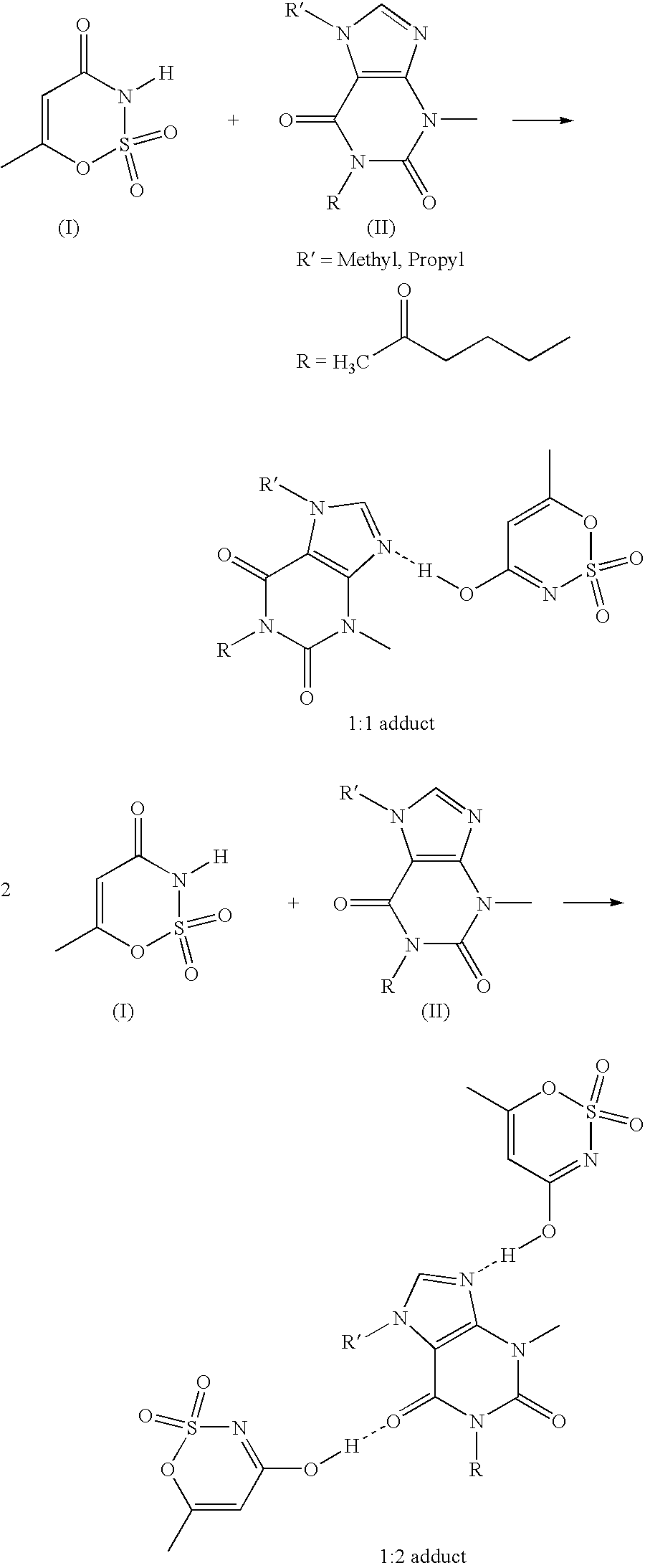
Xanthine-and phenazone-acesulfame-H complexes having improved taste, process for their preparation and their use
Complex compounds or adducts of xanthine derivatives, for example propentofylline or pentoxyfylline, or phenazone derivatives, for example phenazone, propylphenazone and aminophenazone, and acesulfame-H, in which the components are present in a molar ratio of 1:1 or 1:2, have a pleasantly sweet taste and are suitable for numerous applications, for example in pharmaceuticals. The compounds can be prepared from the dissolved components by simple reaction.
Owner:NUTRINOVA NUTRITION SPECIALTIES & FOOD ENGREDIENTS GMBH
Substituted xanthine derivatives
This invention relates to novel compounds that are substituted xanthine derivatives and pharmaceutically acceptable salts thereof. For example, this invention relates to novel substituted xanthine derivatives that are derivatives of pentoxifylline. This invention also provides compositions comprising one or more compounds of this invention and a carrier and the use of the disclosed compounds and compositions in methods of treating diseases and conditions for which pentoxifylline and related compounds are beneficial.
Owner:CONCERT PHARMA INC
Xanthine and phenazone-acesulfame-H complexes having improved taste, process for their preparation and their use
Owner:NUTRINOVA NUTRITION SPECIALTIES & FOOD ENGREDIENTS GMBH
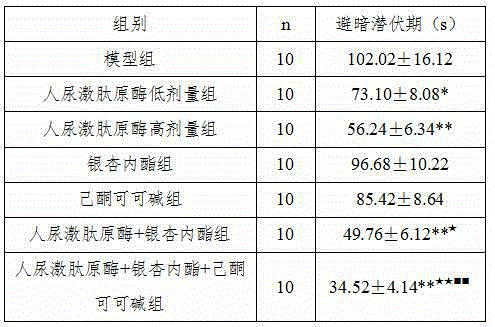
New application of human urine kininogenase and medicine composition comprising same
ActiveCN104940914ADelay disease progressionRestore memoryNervous disorderPeptide/protein ingredientsDiseasePentoxyfylline
The invention belongs to the field of medicine, and particularly relates to a new application of human urine kininogenase and a medicine composition comprising the human urine kininogenase. The human urine kininogenase is applied to prepare medicine for treating the Alzheimer's disease. The invention further provides the medicine composition for treating the Alzheimer's disease. The medicine composition comprises the human urine kininogenase and bilobalide, and further comprises pentoxifyline. The medicine composition can remarkably recover the memory capacity of a patient suffering with the Alzheimer's disease, improve the cognition capacity of the patient suffering with the Alzheimer's disease, delay the disease progress of the Alzheimer's disease, greatly improve the life quality of the patient suffering with the Alzheimer's disease, improve the treatment compliance of the Alzheimer's disease, improve the treatment effect, provide the effective treatment medicine for the patient suffering with the Alzheimer's disease, and greatly relieve pain of the patient of the Alzheimer's disease.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
A kind of carnosine glycoside injection pharmaceutical composition and preparation method thereof
InactiveCN102266351AIncrease breathing vitalityStimulus index increasedPharmaceutical delivery mechanismUnknown materialsDiseaseSodium phosphates
The invention provides a muscular amino acids and nucleosides injection medicinal composition and discloses a preparation method of the medicinal composition. The medicinal composition is prepared from a muscular amino acids and nucleosides injection, pentoxifylline, fructose diphosphate sodium and polyethylene glycol. When the medicinal composition is applied in vitro, the liver homogenate breathing activity of a guinea pig can be increased, an activity stimulation index of breathing activity determined by using a Warburgs respirometer is about 4.2, which is far higher than the stimulation index of the liver homogenate breathing activity of the guinea pig during separate testing by using muscular amino acids and nucleosides, the pentoxifyllinum and the fructose diphosphate sodium, and thus, the treating effect on heart cerebrovascular disease is enhanced greatly.
Owner:长春白求恩制药有限公司
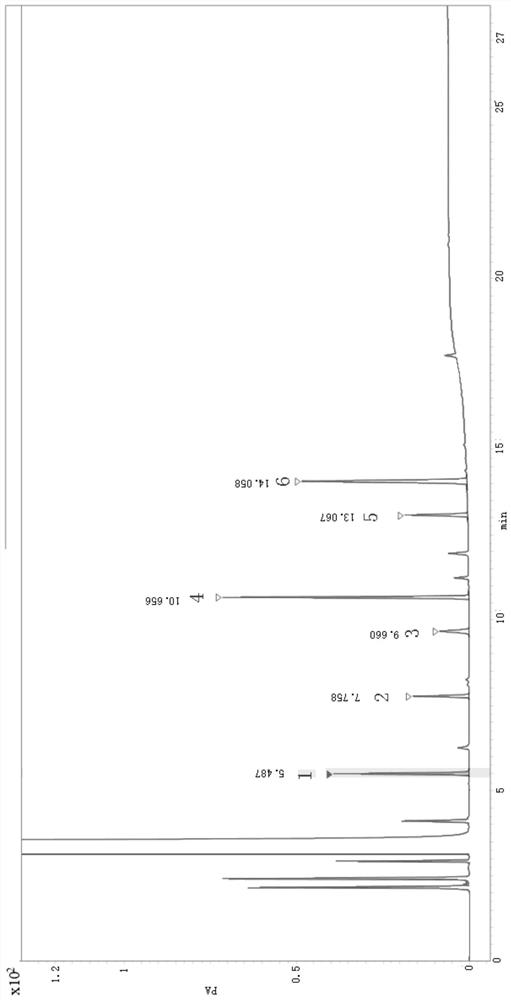
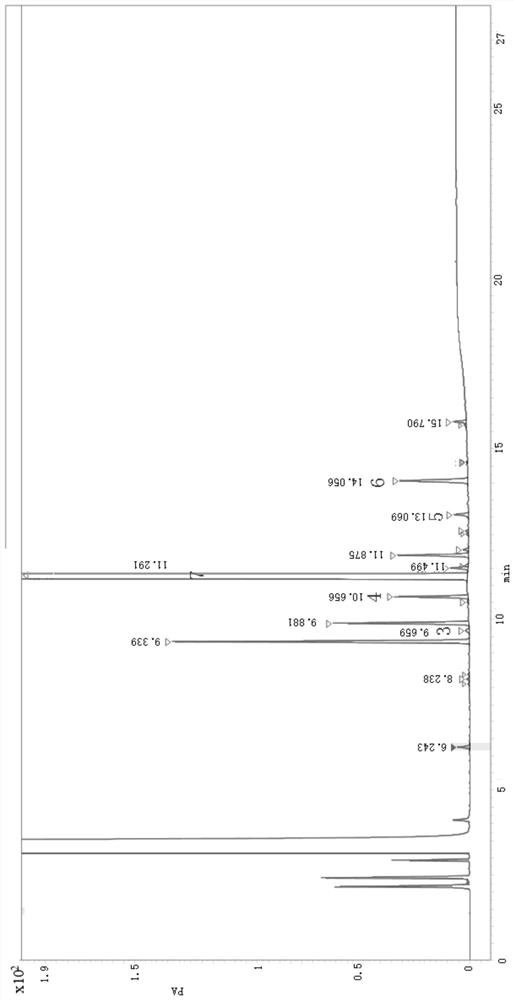
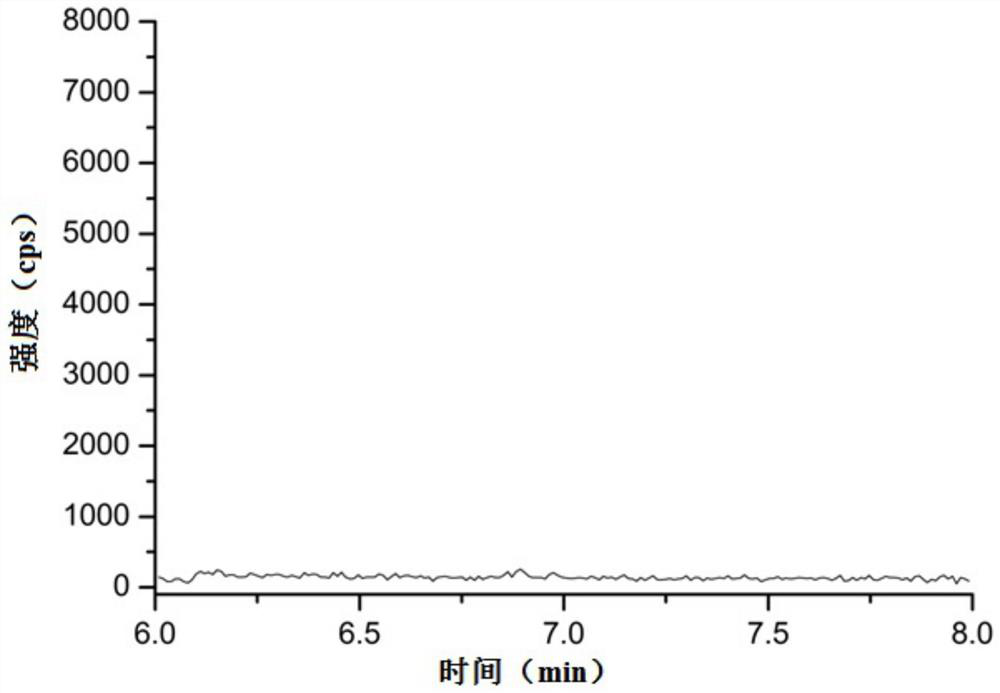
Method for detecting related substances of 6-chloro-2-hexanone
ActiveCN113466353AEasy to separateEnsure quality stabilityComponent separationPentoxyfyllinePhysical chemistry
The invention relates to the field of analytical chemistry, in particular to a method for detecting related substances of 6-chloro-2-hexanone. The detection method adopts a gas chromatographic method for determination, and chromatographic conditions are as follows: a stationary liquid of a capillary chromatographic column is polyethylene glycol or modified polyethylene glycol; and in a heating procedure, the initial temperature is 45-55 DEG C, the temperature is increased to 220-240 DEG C at the speed of 5-15 DEG C / min, and the temperature is maintained for 5-10 min. The detection method provided by the invention is good in sensitivity, accuracy, precision, reproducibility, recovery rate and stability, and accurate and reliable in detection result, and provides a guarantee for monitoring the quality stability and clinical medication safety of pentoxifylline synthesized by taking 6-chloro-2-hexanone as a starting material.
Owner:沧州临港友谊化工有限公司
New application of human urinary kallidinogenase and pharmaceutical composition with the human urinary kallidinogenase
InactiveCN104906564ARelieve painGood blood pressure effectPeptide/protein ingredientsPharmaceutical delivery mechanismSide effectEfficacy
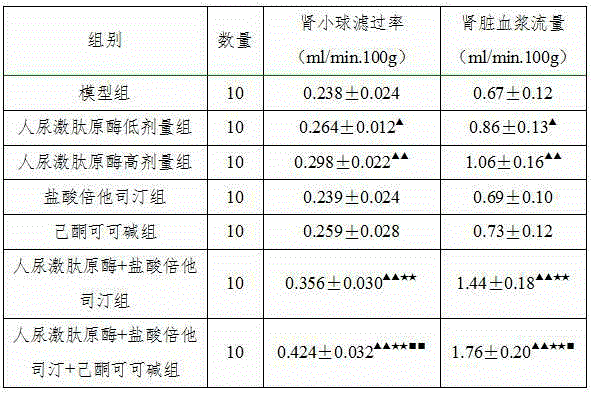
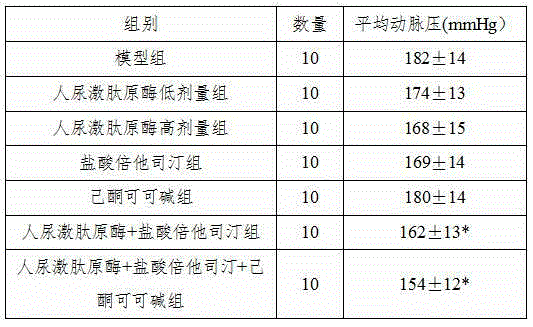
The invention belongs to the field of medicine, in particular to a new application of human urinary kallidinogenase and a pharmaceutical composition with the human urinary kallidinogenase. According to the new application of the human urinary kallidinogenase, the human urinary kallidinogenase is used for preparing medicine for treating hypertensive nephropathy, and the test discovers that the human urinary kallidinogenase is capable of lowering the arterial pressure of a rat with hypertensive nephropathy and improving the glomerular filtration rate and renal plasma flow of the rat with hypertensive nephropathy through regulating the micro-circulation of glomerulus, and has a certain treatment effect for renal damage. The pharmaceutical composition for treating the hypertensive nephropathy comprises the human urinary kallidinogenase and betahistine hydrochloride and further comprises pentoxifylline, the pharmaceutical composition has an obvious blood pressure reducing efficiency and has an obvious treatment effect for the damaged kidney, and the pharmaceutical composition for treating the hypertensive nephropathy is definite in effect, low in side effect and better for the recovery of a patient with hypertensive nephropathy.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
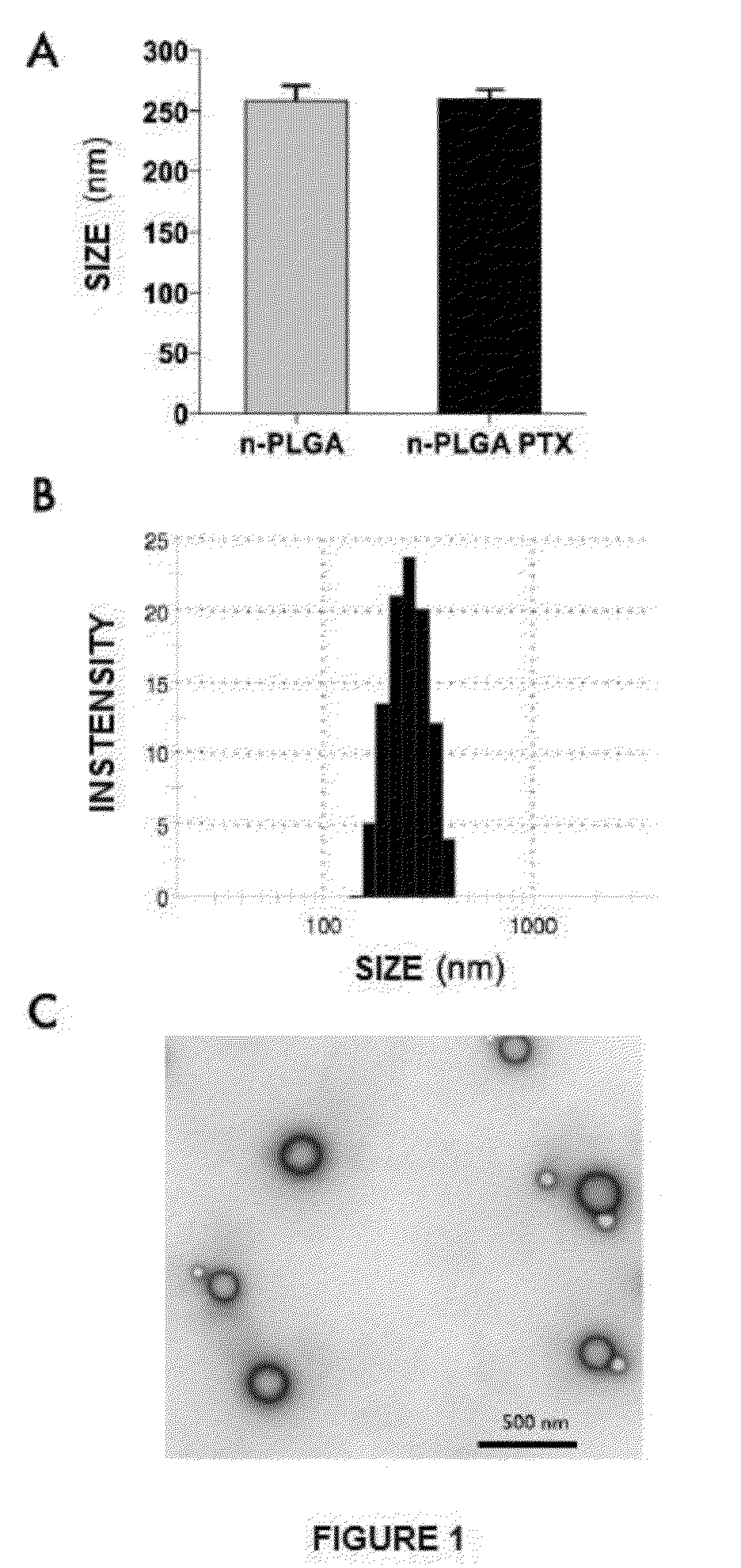
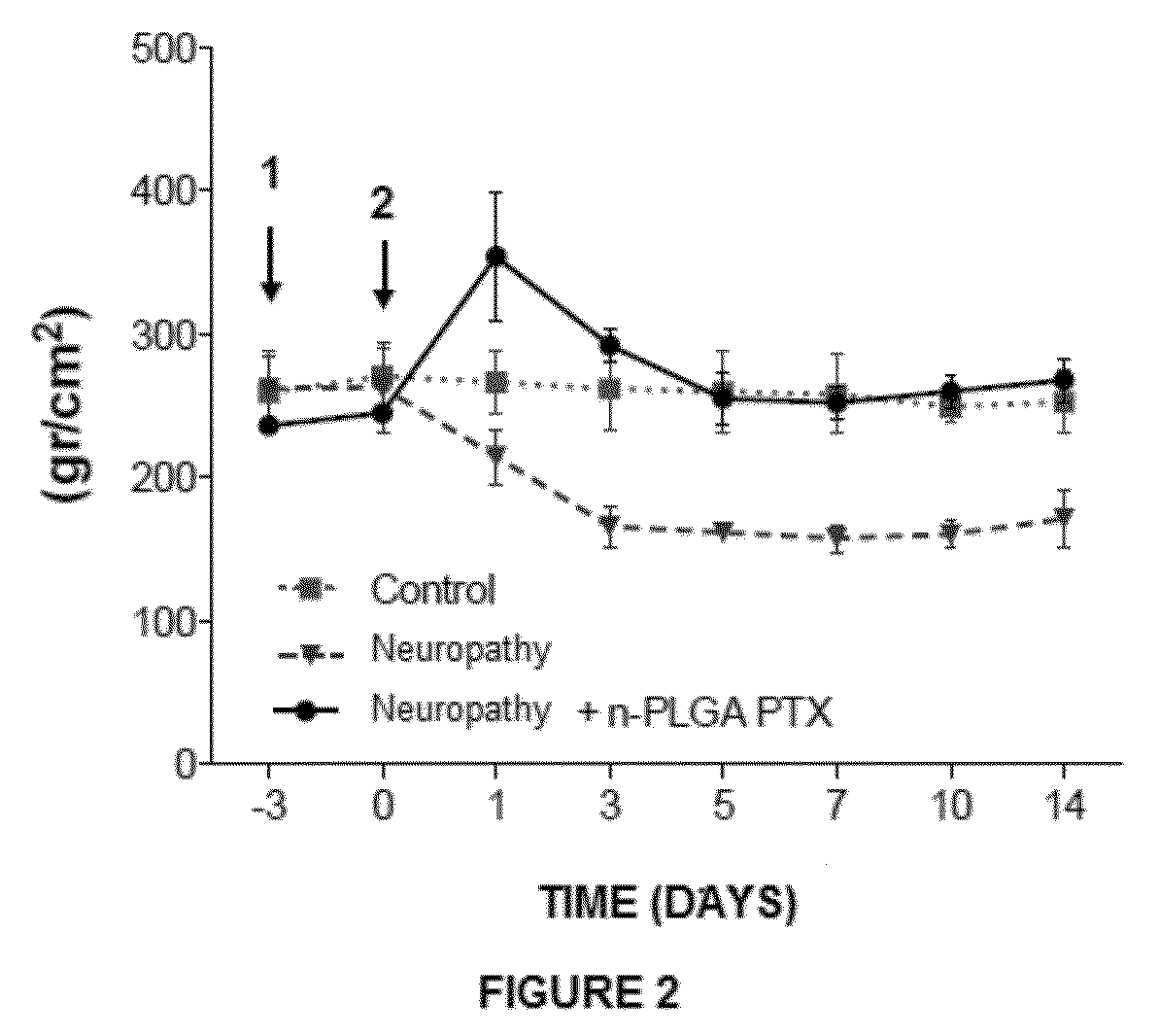
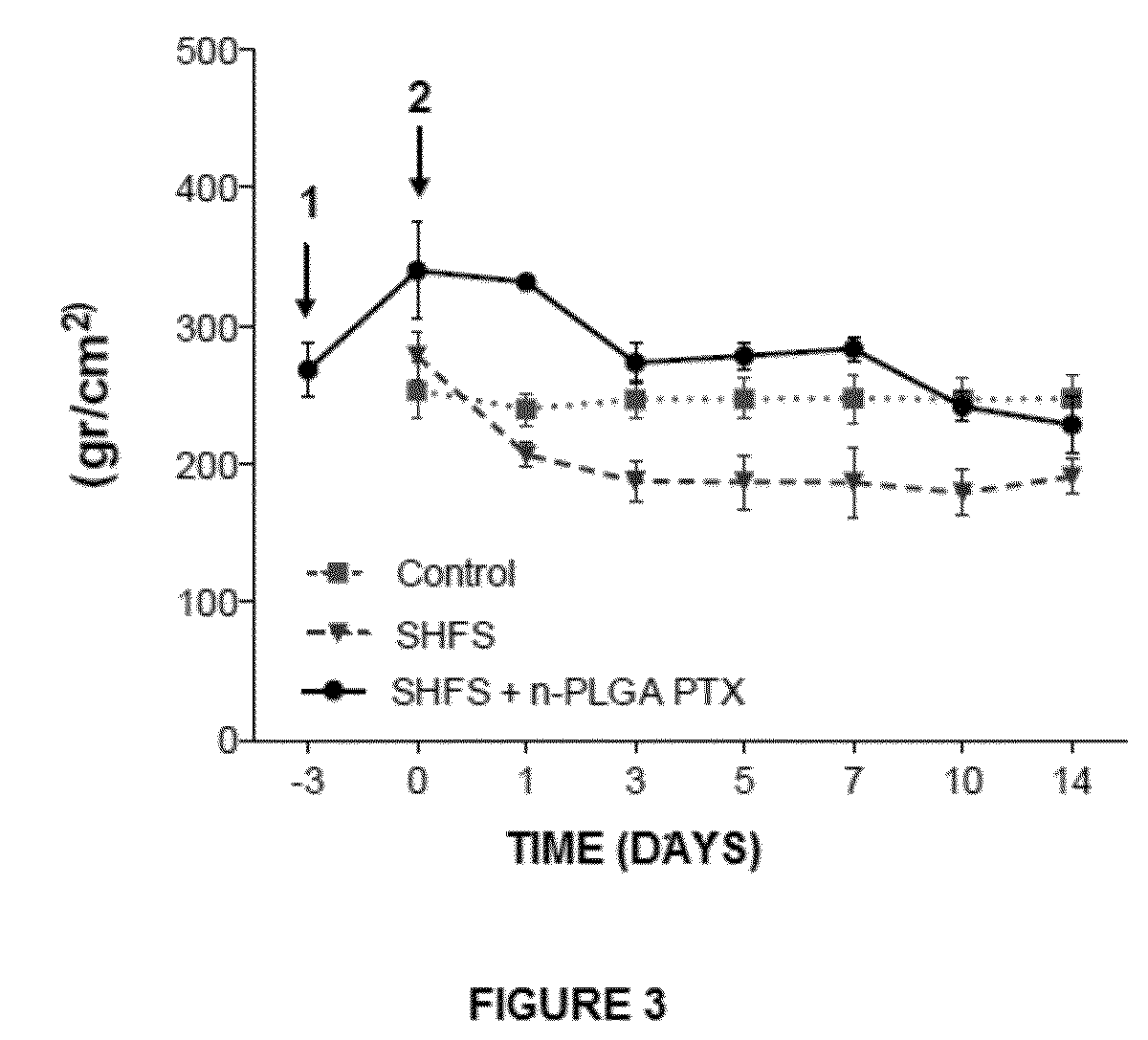
Nanoparticles with biodegradable and biocompatible polymer plga, loaded with the drug for human use pentoxifylline
The invention relates to a novel pharmaceutical formulation comprising polymer nanoparticles of the biodegradable and biocompatible polymer poly(lactic-glycolic) acid (PLGA), loaded with the drug pentoxifylline, the method for the synthesis of the PLGA nanoparticles loaded with pentoxifylline, and to the use thereof in the effective treatment for the relief of chronic pain and for the prevention of chronic pain via the administration of a single dose.
Owner:UNIV DE SANTIAGO DE CHILE
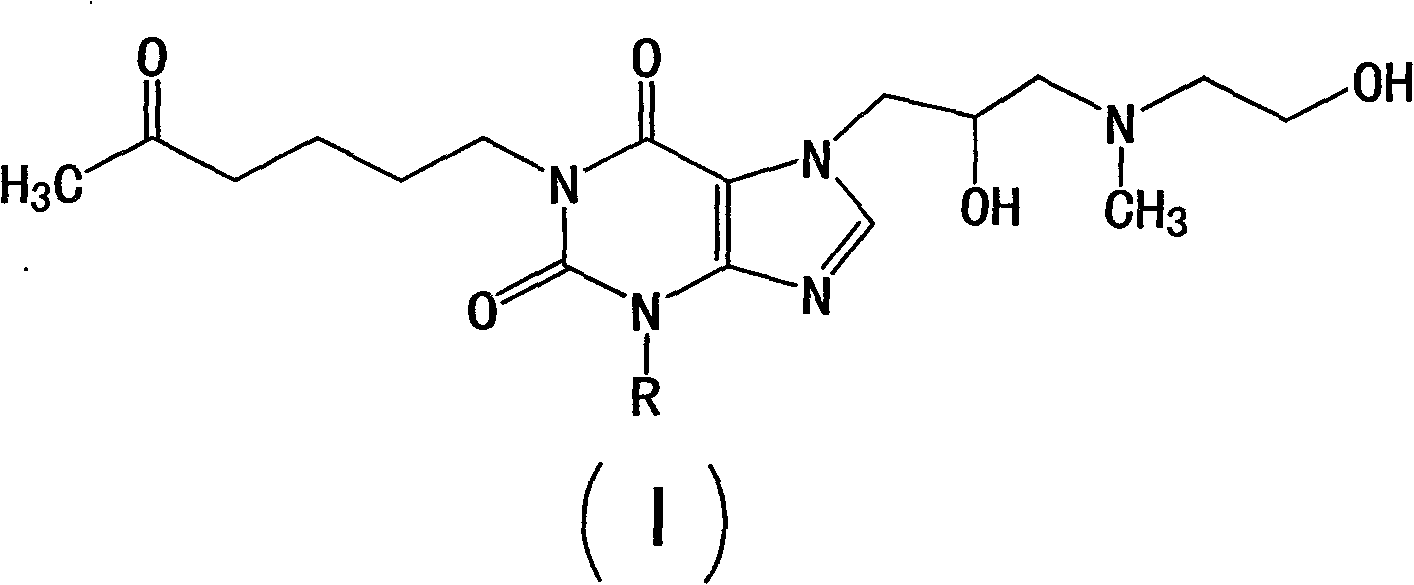
Pentoxifylline derivative
InactiveCN101648951AGood vasodilation and neuroprotectionImprove protectionNervous disorderOrganic chemistryNeuroprotectionTheobromine
The invention discloses a pentoxifylline derivative shown as the formula (1) or pharmaceutically acceptable salt, solvate, optical isomer or polymorph and a pharmaceutical composition containing the compounds, wherein R represents straight chain or branch chain C1-C5 alkyl, C3-C6 naphthenic base or C4-C8 naphthenic alkyl. The invention also discloses a preparation method of the pentoxifylline derivative and application in preparing a medicament with nerve protection function. The compound has favorable effects of hemangiectasis and nerve protection and higher application value in the field ofmedicines.
Owner:徐奎
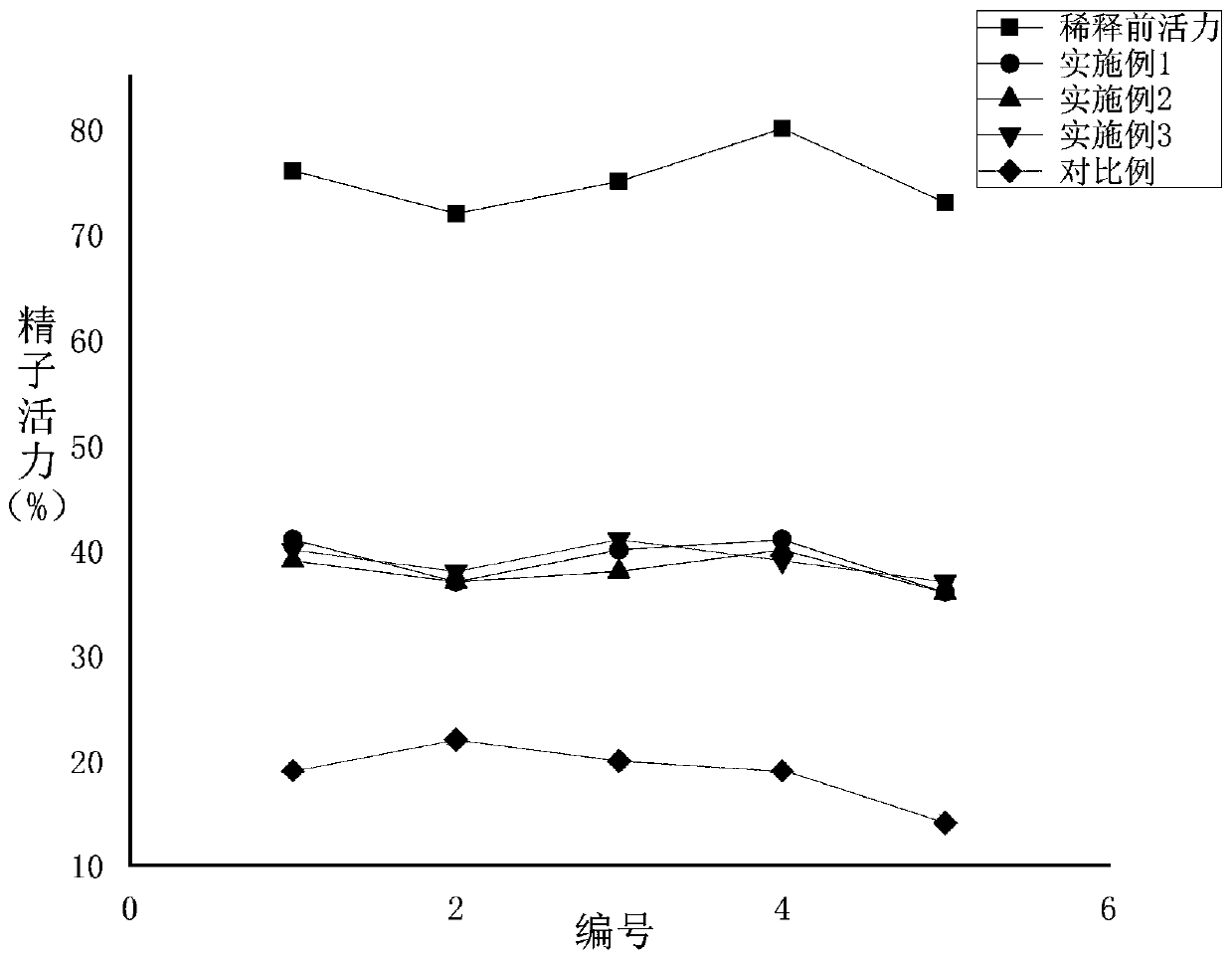
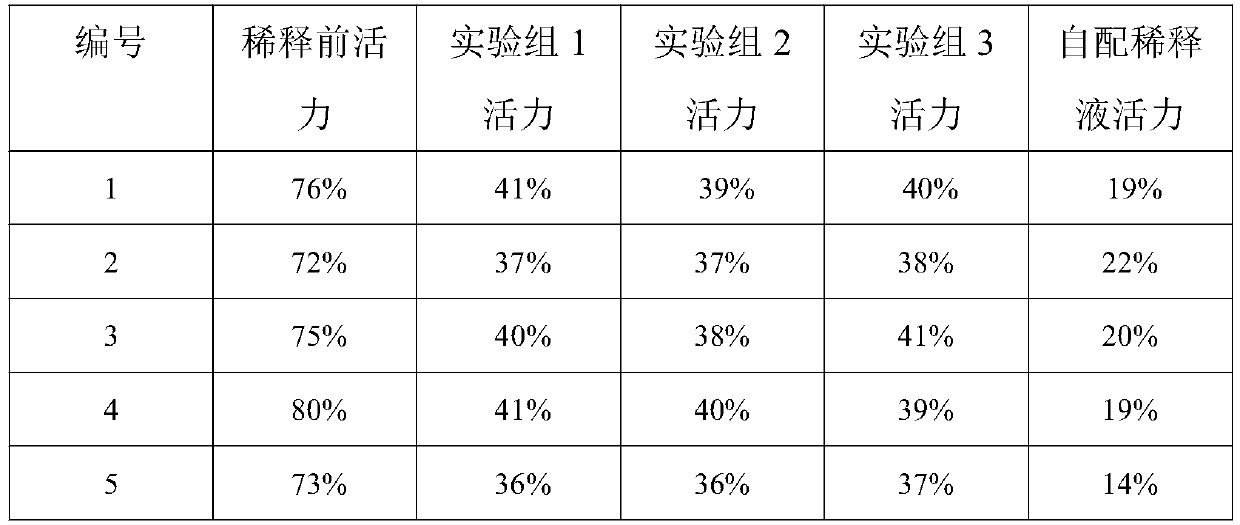
Sperm diluent for donkeys and preparation method and application thereof
InactiveCN111493062AAvoid harmReduce electrolyte concentrationDead animal preservationPhysiologyPhosphorylation
The invention discloses sperm diluent for donkeys. The invention discloses a donkey sperm diluent and a preparation method thereof. The composition comprises the following components: 30.4+ / -0.5g of tris, 17.0+ / 0.2 g of citric acid; 7.5+ / -0.1 g of glucose, 12.5+ / -0.2 mg of taurine, 36.3+ / -0.5 mg of sodium pyruvate, 1.5+ / -0.1 g of fructose, 2+ / -0.1 g of modified lecithin, 1.5+ / -0.1 g of pentoxifylline and 25.4+ / -0.3 mg of soy isoflavone which are dissolved with water for injection. By adopting the modified lecithin, the damage of irregular particles in common phospholipid to sperms is avoided,the effects of regulating osmotic pressure and protecting sperm cell membranes are achieved, and the survival rate of the sperms is increased; pentoxifylline is adopted, so that the concentration of cAMP in sperm cytoplasm is increased, cAMP dependent protease is activated, tail parts of sperms are phosphorylated, and the athletic ability of the sperms is enhanced; soybean isoflavone is adopted, excessive free oxygen of sperms in an in-vitro environment is removed, oxidative damage to the sperms is avoided, sperm motility is maintained, and the success rate of artificial insemination is increased.
Owner:太东(镇江)生物科技有限公司
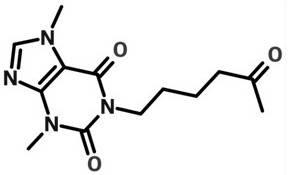
Pentoxifylline injection and preparation method thereof
ActiveCN113069411AImprove stabilityReduce adverse effectsInorganic non-active ingredientsPharmaceutical delivery mechanismPentoxyfyllineTheobromine
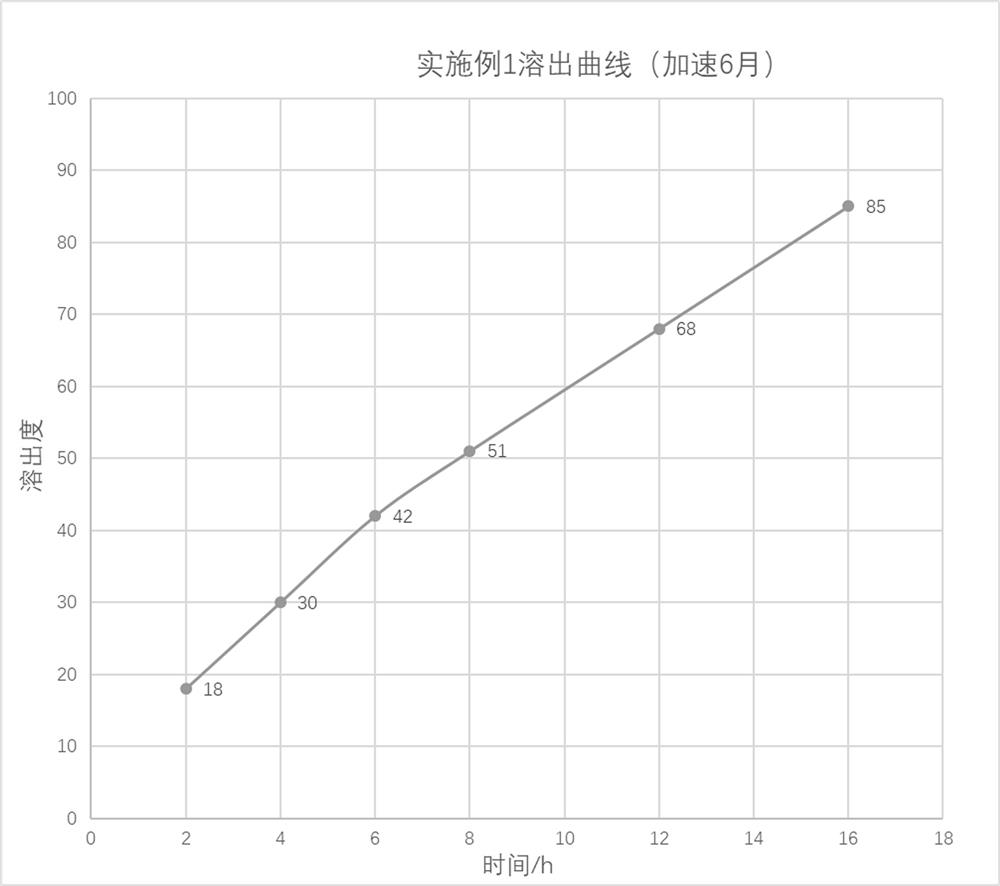
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses a pentoxifylline injection and a preparation method thereof. The preparation method comprises the following steps of adding the osmotic pressure regulator of the prescription dosage into the water for injection which is 40-60% of the total preparation volume, stirring and dissolving, adjusting the pH value to 6.5-7.5, then adding the pentoxifylline of the prescription dosage, stirring and dissolving, supplementing the water for injection to the total preparation volume, adjusting the pH value to 6.5-7.5, filtering through a multi-stage polyether sulfone filter element of which the pore diameter is sequentially reduced, filling, and sterilizing to obtain the pentoxifylline injection. Compared with the prior art, the preparation method realizes the purpose of ensuring the product quality stability under the condition of not adding a stabilizer, the prescription is simpler, the total impurity content is less than or equal to 0.05%, the impurity content is basically not increased, the pH value is stable, the solution is always a colorless and clear solution in an accelerated six-month test process, the production cost is also reduced, the industrial production is convenient to realize, and the popularization value is relatively high.
Owner:SHIJIAZHUANG NO 4 PHARMA
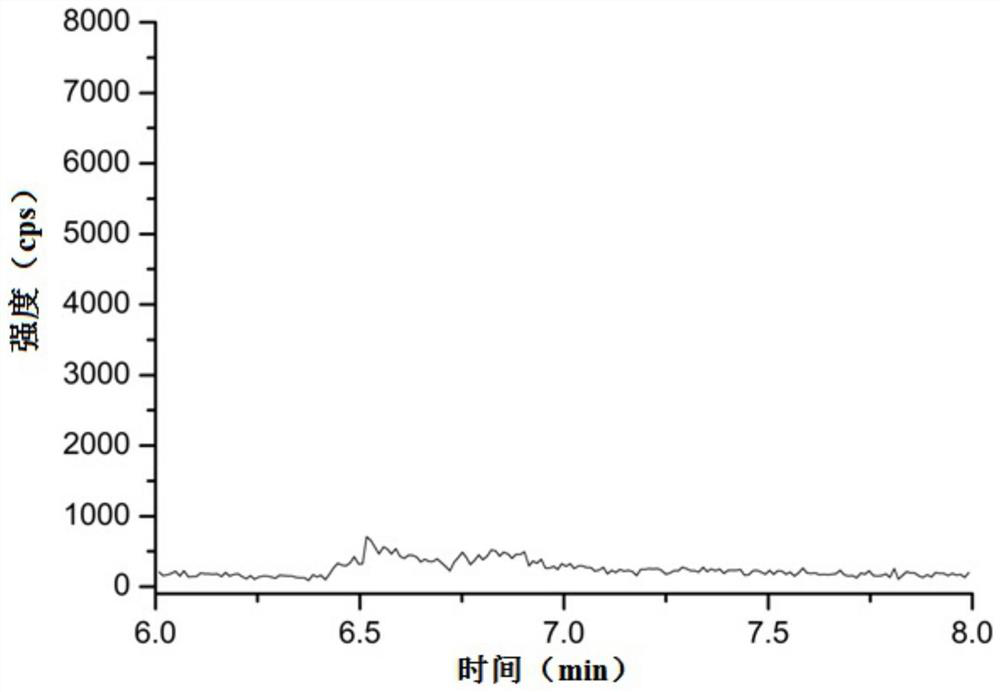
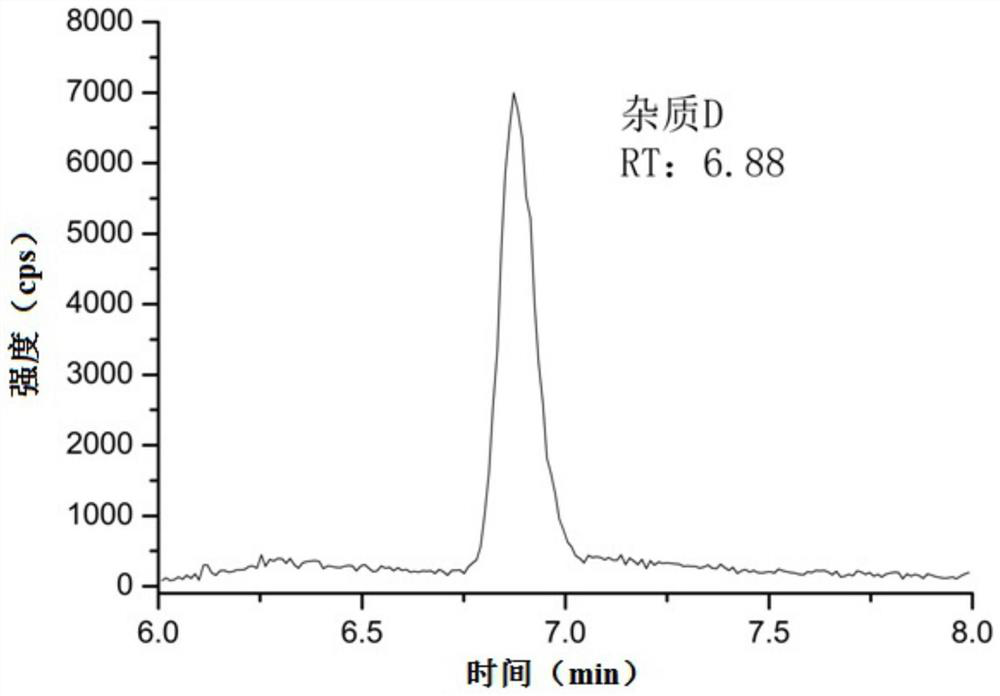
Method for detecting genotoxic impurity in pentoxifylline
ActiveCN113686981AQuantitativeRealize qualitative analysisComponent separationLiquid chromatography mass spectroscopyGradient elution
The invention relates to the technical field of pharmaceutical analysis, and particularly discloses a method for detecting a genotoxic impurity in pentoxifylline. The detection method comprises the following steps that: a test solution and a reference solution are prepared; and the test solution and the reference solution are detected by adopting a liquid chromatography-mass spectrometry method. The chromatographic conditions of liquid chromatography are as follows: gradient elution is performed by adopting a C18 chromatographic column and taking a formic acid aqueous solution with the volume concentration of 0.1% as a mobile phase A and methanol as a mobile phase B; and the mass spectrometry adopts an ESI ion source and a positive ion detection mode. The detection method provided by the invention has the advantages of simplicity, stability, high precision, good reproducibility and the like, can quickly and accurately detect a genotoxic impurity D in a pentoxifylline bulk drug, and accords with the guiding principle of ICH M7.
Owner:SHIJIAZHUANG NO 4 PHARMA
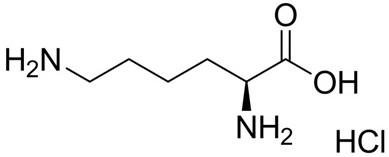
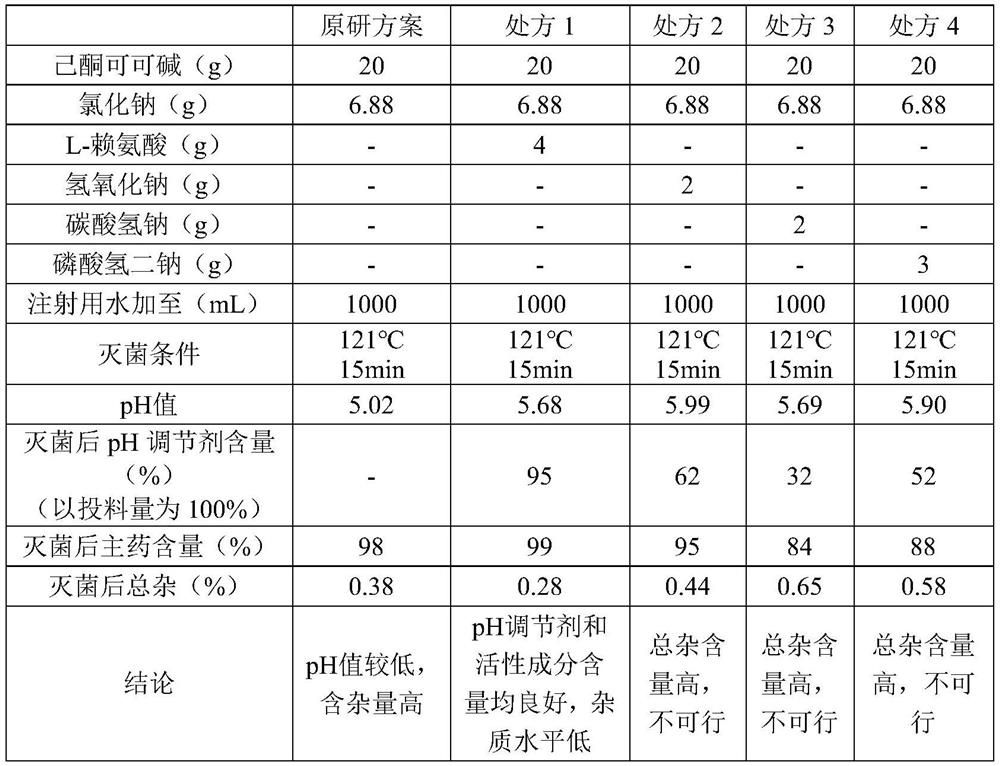
Preparation of pH-stable pentoxifylline injection
InactiveCN112842993AStable pHGood effectInorganic non-active ingredientsPharmaceutical delivery mechanismDiseasePentoxyfylline
The invention discloses a prescription and a preparation method of a pH-stable pentoxifylline injection. The prescription is composed of pentoxifylline, sodium chloride, L-lysine and water for injection, and the dosage of the L-lysine is 3-7 mg / ml. After the L-lysine (pH 5.5-6.0) is added, the pH value of the pentoxifylline injection is more stable, and the L-lysine serving as a brain protective agent for systemic brain trauma, chronic tissue ischemia and ischemic diseases has a synergistic effect with the pentoxifylline, so that the effect of pentoxifylline for improving the blood flow volume of the brain and improving nutritional microcirculation of the body and oxygen supply of an ischemic area are improved. The pentoxifylline injection disclosed by the invention is simple and efficient in prescription process and good in safety.
Owner:南京泽恒医药技术开发有限公司
A kind of refining method of pentoxifylline recovered product
The invention discloses a method for purifying a recovered pentoxifylline product, which can reduce the impurity content in the recovered pentoxifylline product, so that the maximum single impurity of the recovered product after being refined is reduced to less than 0.1%, and the total impurity is less than 0.5%, which satisfies The standard of finished pentoxifylline can improve the overall yield and reduce the reaction cost. The refining method comprises the following steps: (1) adding the pentoxifylline recovered product into water under heating conditions, dissolving, adding an alkaline liquid to adjust the pH value to 10-14, adding a reducing agent, keeping the temperature, and then cooling and filtering to obtain an alkaline solution; Wherein, in the pentoxifylline recovered product, mono-impurities < 5%, total impurities < 10%; (2) fully mixing the alkaline solution obtained in step (1) with organic solvent A, leaving it to stand for stratification, and steaming off the organic phase Then obtain a viscous liquid; (3) add organic solvent B to dissolve the above-mentioned viscous liquid, add activated carbon, filter the activated carbon after heat preservation, filter the material after the filtrate is reduced to below 20 DEG C, and dry to obtain pentoxifylline.
Owner:CSPC INNOVATION PHARMA
Nano-carrier sustained release preparation for treating cardiovascular diseases and preparation method of nano-carrier sustained release preparation
ActiveCN114053236AGuaranteed therapeutic effectDissolution rate is stablePowder deliveryPill deliveryCelluloseCholic acid
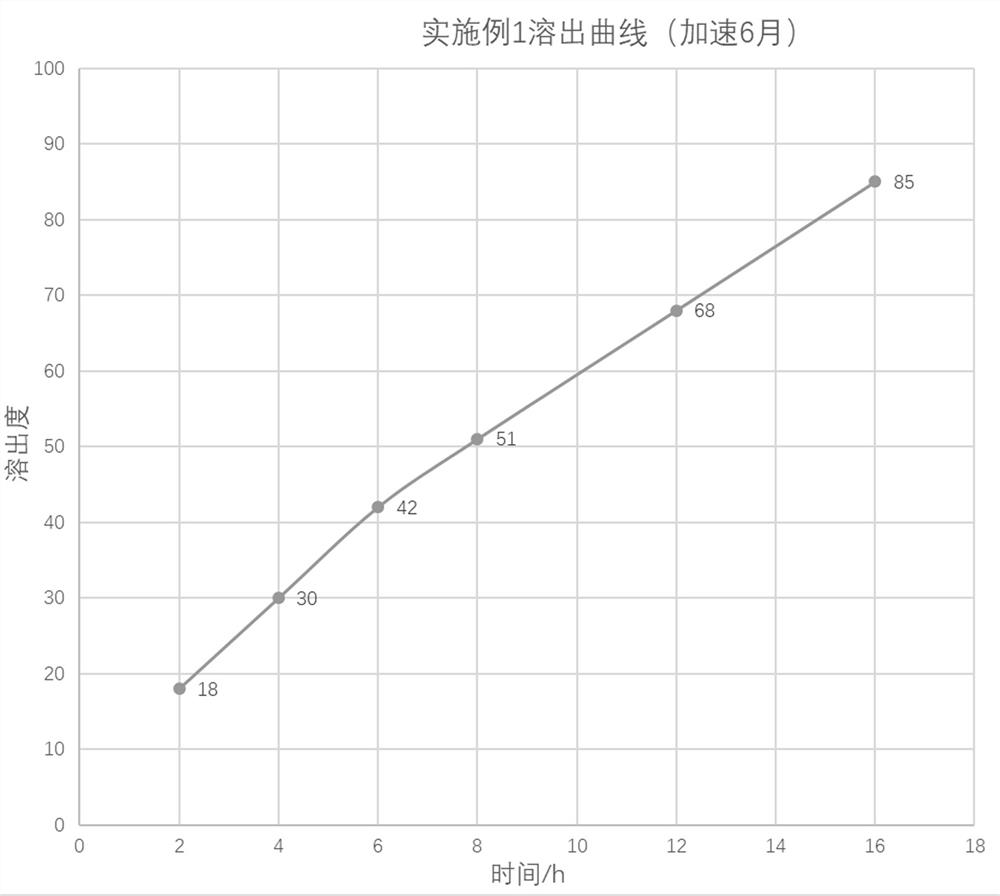
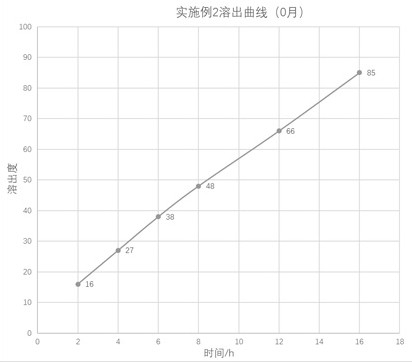
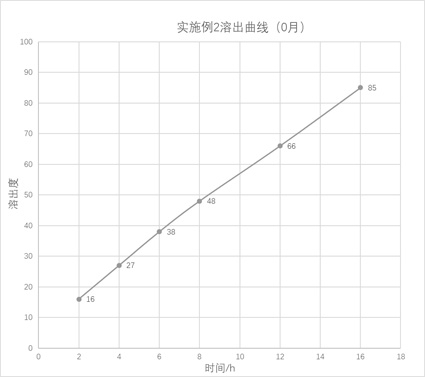
The invention provides a nano-carrier sustained release preparation for treating cardiovascular diseases and a preparation method of the nano-carrier sustained release preparation. The nano-carrier sustained release preparation consists of freeze-dried pentoxifylline nano-carrier powder and pharmaceutic adjuvants. A weight ratio of pentoxifylline to a nano-carrier in the freeze-dried pentoxifylline nano-carrier powder is (4-5): 1; the nano-carrier is made of a composition of chitosan oligosaccharide, deoxycholic acid and albumin, wherein a weight ratio of chitosan oligosaccharide to deoxycholic acid to albumin is 1: (2-3): (3.0-3.3); a weight ratio of pentoxifylline to the pharmaceutic adjuvants is (4-7): 1; and the pharmaceutic adjuvants are composed of hydroxyethyl cellulose, aerosil and magnesium stearate, wherein a weight ratio of hydroxyethyl cellulose to aerosil to magnesium stearate is (5-6): (3-4): 1. The nano-carrier sustained release preparation for treating cardiovascular diseases provided by the invention is stable in dissolution speed, basically realizes zero-order linear dissolution, avoids burst release, greatly ensures the treatment effect of pentoxifylline, and obviously reduces adverse reactions; and after being placed for 6 months under an accelerated condition, the content of a degradation product is low, and stable quality is realized.
Owner:HEBEI CHEM & PHARMA COLLEGE
Prepn. for stopping vomitus contg. compound pentoxifylline
InactiveCN1513455AGood curative effectReduce adverse reactionsMetabolism disorderUrinary disorderTheobromineBiomedical engineering
A compound trental-sinemet medicine is prepared from sinemet or physiologically receptable salt and trental. Its medical usage is also disclosed.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Blood vessel blocking agent bond joint fluorine-boron pyrrole derivative and preparation method and application thereof
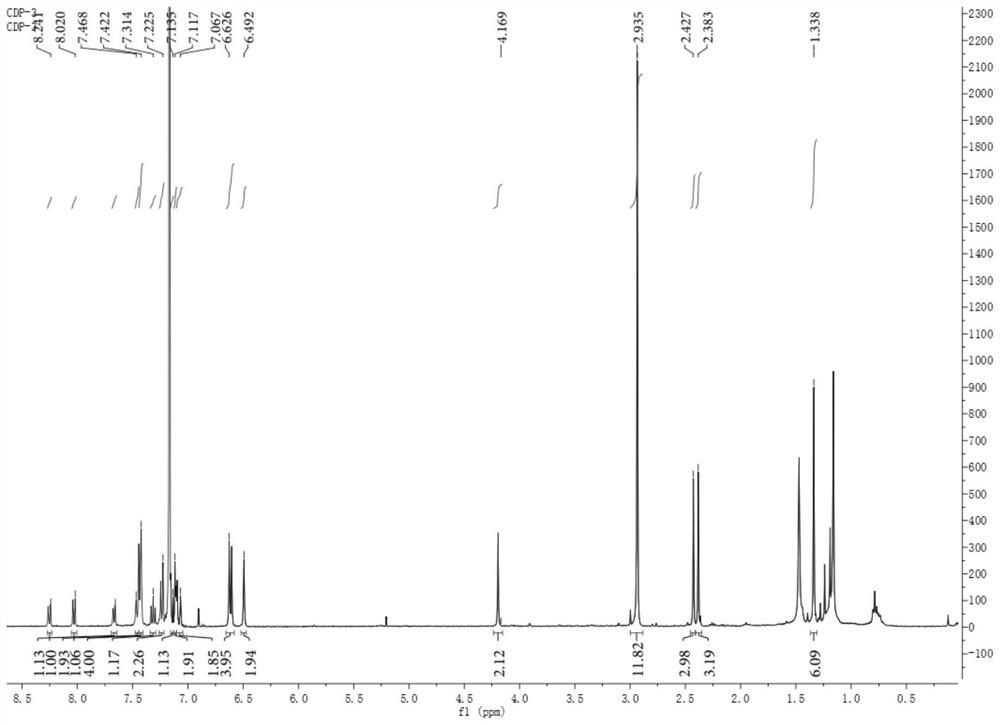
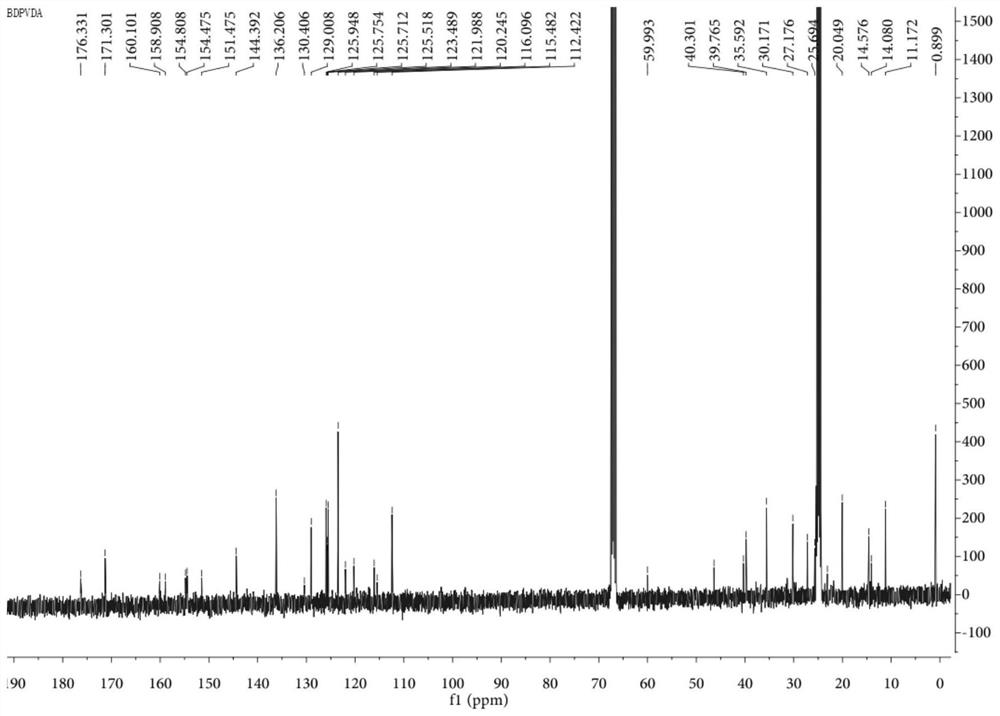
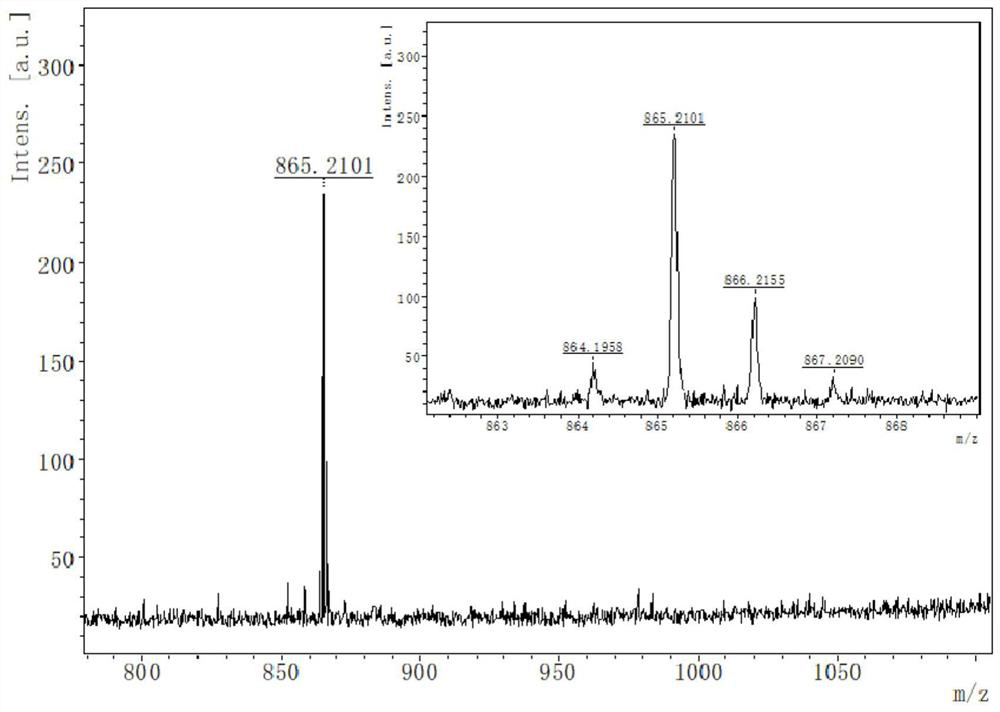
ActiveCN111084881AClear structureThe synthesis method is simpleOrganic active ingredientsPhotodynamic therapyTumor therapyMethacrylate methyl
The invention discloses a blood vessel blocking agent bond joint fluorine-boron pyrrole derivative. The blood vessel blocking agent bond joint fluorine-boron pyrrole derivative is prepared through covalent bond joint of a near-infrared photosensitizer namely fluorine-boron pyrrole and a blood vessel blocking agent namely 2,5-pentoxifylline. Besides, the invention also discloses a nanometer diagnosis and treatment reagent obtained through self-assembling of the derivative and electron-enriched amphiphilic polymer namely methoxy-poly(ethanediol)-poly(2-diisopropylamine) methyl methacrylate, andan application of the nanometer diagnosis and treatment reagent to tumor treatment through synergism of I type photodynamic therapy and a blood vessel blocking treatment method. The blood vessel blocking agent bond joint fluorine-boron pyrrole derivative disclosed by the invention is clear in the structure of objective products, and simple in synthesis technology; the diagnosis and treatment reagent based on the objective products concurrently has I type photodynamic and pH responsiveness blood vessel blocking agent releasing properties, under passive targeting effects and fluorescence imagingmediating, the nanometer diagnosis and treatment reagent can accurately reach tumor positions, block tumor blood vessels and kill tumor cells, so that recurrence and transfer of tumors can be effectively prevented.
Owner:NANJING UNIV OF TECH
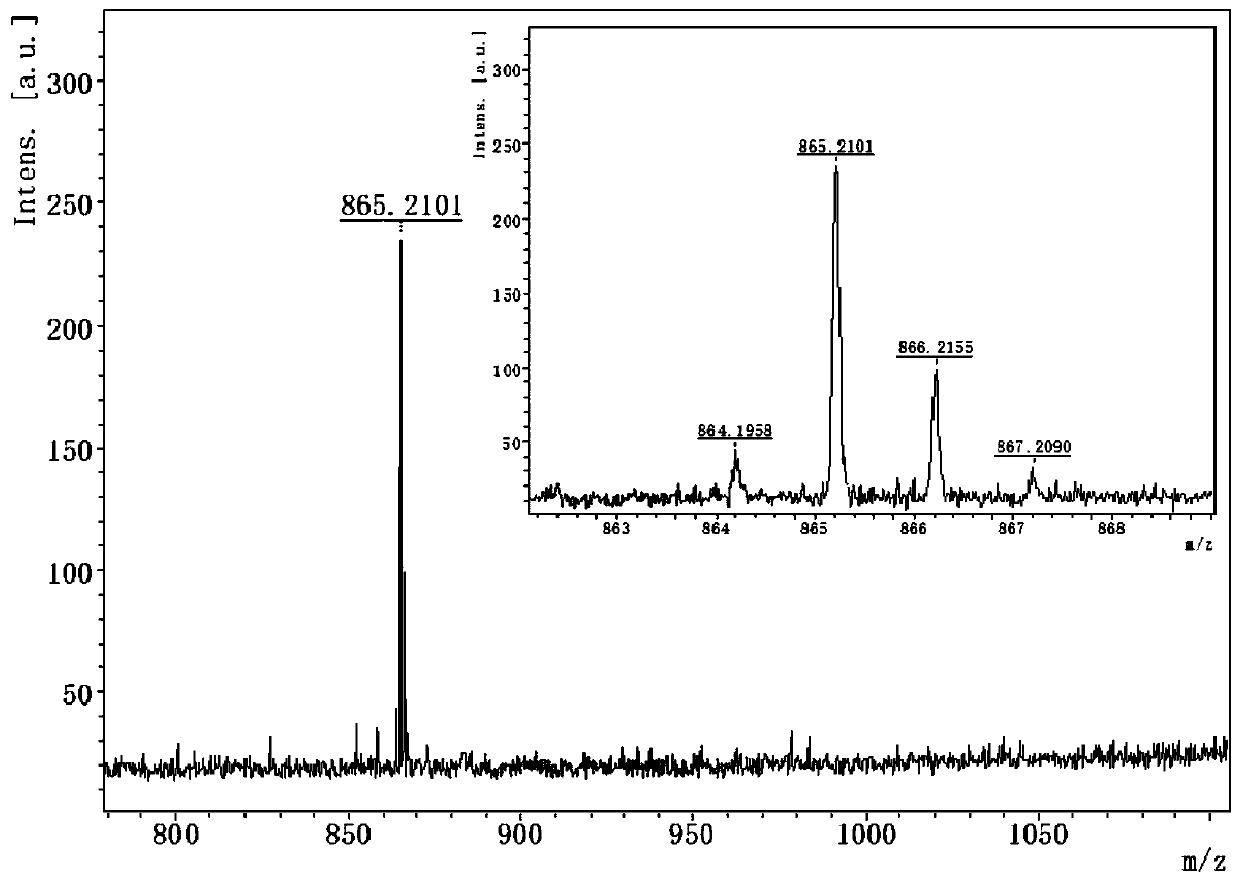
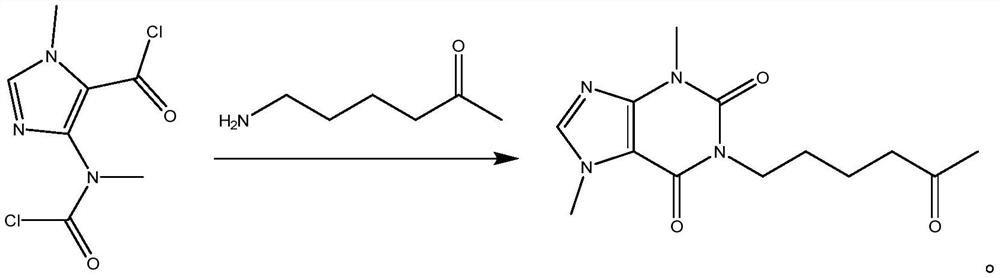
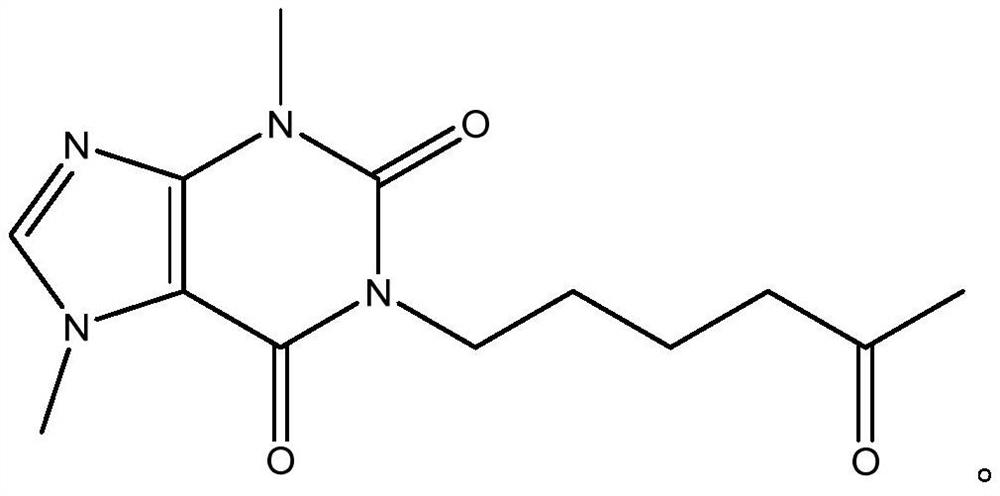
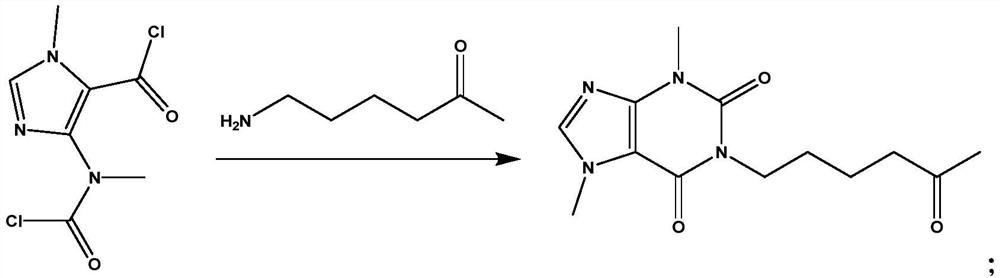
Synthesis method of pentoxifylline
The invention belongs to the field of medicine synthesis, and discloses a synthesis method of pentoxifylline, which is characterized in that 4-(nitrogen methyl acyl chloride)-1-methylimidazole-5-acyl chloride and amino-5-hexanone are subjected to condensation ring closing, and the target product pentoxifylline is synthesized by only one-step reaction. The pentoxifylline synthesis method provided by the invention is simple in synthesis process, and byproducts obtained in the reaction process can be recycled to synthesize pentoxifylline again. The method is suitable for synthesizing pentoxifylline, and the synthesized pentoxifylline is used for preparing pentoxifylline for injection.
Owner:HAINAN GENERAL & KANGLI PHARMA
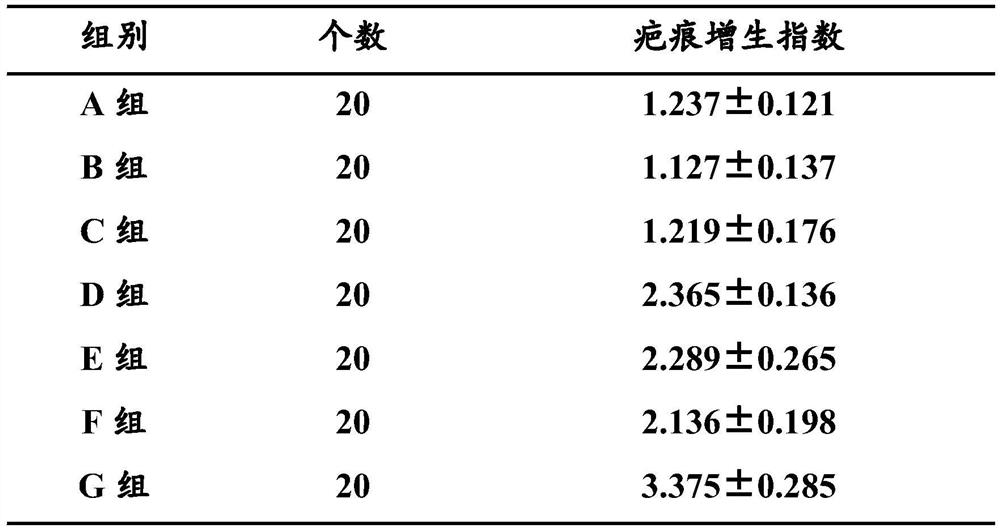
A kind of anti-scar silicone gel patch and preparation method thereof
ActiveCN110917179BInhibitionPromote wound healingPharmaceutical non-active ingredientsDermatological disorderSilicone GelsPentoxyfylline
The invention belongs to the field of medicine, and in particular relates to an anti-scar silicone gel patch and a preparation method thereof. The anti-scar silicone gel patch includes a silicone gel patch and a plastic film; the silicone gel patch includes a silicone gel layer and a textile layer; by weight percentage, the silicone gel layer contains 10-14% Tamoxifen, 6‑10% pentoxifylline, and 75‑85% silicone polymer. Applying tamoxifen and pentoxifylline to silicone gel to make anti-scar silicone gel patch has obvious effect on reducing scar tissue formation. Compared with the lack of any one component, the combined use of the three can play a synergistic effect, can significantly inhibit the proliferation of skin scar cells, and effectively remove scar marks.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
Preparation method of pentoxifylline impurity
The invention discloses a preparation method of a pentoxifylline impurity. The preparation method comprises the following steps: adding theobromine into an organic solvent, stirring, and adding a certain amount of alkali; slowly dropwise adding a halogenating reagent into a reaction system for reaction under a temperature control condition; after the reaction is finished, filtering the reaction liquid, adding alkali liquor into the obtained filtrate to adjust the pH value to 10-11, adding a solvent for extraction, and separating the liquid; and washing, drying, concentrating and purifying the organic phase to obtain a target product. The method is easy to operate and can be used for quickly preparing the high-purity pentoxifylline impurity.
Owner:赤峰经方医药技术开发有限责任公司
Pentoxifylline as well as synthesis method and application thereof
PendingCN114380829ALow toxicityAvoid introducingOrganic chemistryDigestive systemPotassium carbonateSodium hydroxide
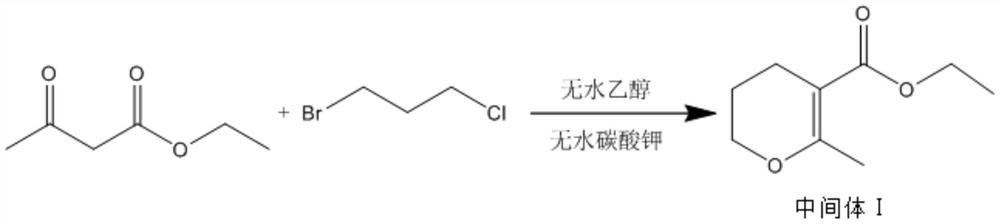
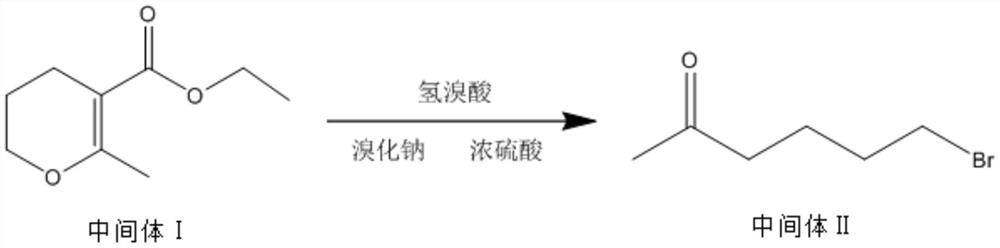
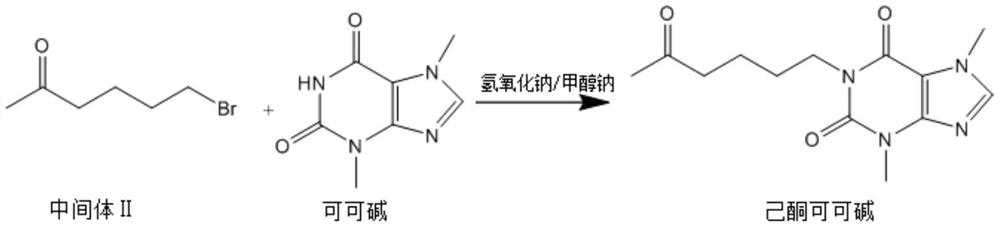
The invention discloses a synthesis method of pentoxifylline, which comprises the following steps: 1, synthesizing an intermediate I: adding 0.5-1.5 parts of 1, 3-bromochloropropane into 6.5-7.5 parts of absolute ethyl alcohol, 2-3 parts of anhydrous potassium carbonate and 1-2 parts of ethyl acetoacetate to carry out heating reflux reaction, and separating and purifying the reaction product to obtain the intermediate I; step 2, synthesizing an intermediate II: adding 0.5-1.5 parts of the intermediate I, 0.5-1.0 part of sodium bromide and 1-2 parts of 40-60% hydrobromic acid into the reaction container, dropwise adding concentrated sulfuric acid under a low-temperature condition until heat release is violent, carrying out heating reflux reaction, and separating and purifying a reaction product to obtain the intermediate II; and step 3, synthesizing pentoxifylline, namely dissolving 0.5-1.5 parts of theobromine in 2-3 parts of 10-30% sodium hydroxide solution, dropwise adding a mixed solution of 0.5-1.5 parts of the intermediate II and 4-5 parts of methanol, carrying out heating reflux reaction, and separating and purifying a reaction product to obtain the pentoxifylline. The synthesis process disclosed by the invention is relatively low in environmental pollution, and the synthesized pentoxifylline is relatively high in purity.
Owner:赤峰万泽药业股份有限公司
Compositions and methods of administering a colchicine based topical composition for the prevention of radiation fibrosis
ActiveUS10772813B2Avoid scaringInhibition formationCosmetic preparationsToilet preparationsPentoxyfyllineFibrosis
A colchicine-containing composition comprising colchicine as the active ingredient or colchicine in combination with pentoxifylline and tocopherol (Vitamin E) which are formulated for topical use in the prevention and treatment of radiation-induced fibrosis. And methods of making and administering the colchicine-containing compositions. The compositions can be used as topical applications for the prevention and treatment of radiation-induced fibrosis, commonly known as scarring, that can be debilitating, and can occur as a late and permanent complication of radiation therapy.
Owner:COLRADEL LLC
A nano-carrier slow-release preparation for treating cardiovascular disease and its preparation method
ActiveCN114053236BGuaranteed therapeutic effectDissolution rate is stablePowder deliveryPill deliveryCelluloseCholic acid
The invention provides a nano-carrier slow-release preparation for treating cardiovascular disease and a preparation method thereof, which is composed of pentoxifylline nano-carrier freeze-dried powder and pharmaceutical auxiliary materials. Described pentoxifylline nano-carrier lyophilized powder, the weight ratio of pentoxifylline and nano-carrier is 4~5:1; Described nano-carrier material is the composition of chitosan-deoxycholic acid-albumin, wherein The weight ratio of chitooligosaccharide, deoxycholic acid and albumin is 1:2-3:3.0-3.3; the weight ratio of the pentoxifylline and the pharmaceutical excipient is (4-7):1, the drug The excipients are composed of hydroxyethyl cellulose, micropowder silica gel and magnesium stearate, wherein the weight ratio of hydroxyethyl cellulose, micropowder silica gel and magnesium stearate is (5-6):(3-4):1. The dissolution rate of the sustained-release nanocarrier preparation for the treatment of cardiovascular provided by the present invention is stable, basically showing zero-order linear dissolution, avoiding sudden release, greatly ensuring the therapeutic effect of pentoxifylline, and significantly reducing adverse reactions; placed under accelerated conditions After 6 months, the content of degradation products is low and the quality is stable.
Owner:HEBEI CHEM & PHARMA COLLEGE
A kind of pentoxifylline injection and preparation method thereof
ActiveCN113069411BImprove stabilityReduce adverse effectsInorganic non-active ingredientsPharmaceutical product form changePentoxyfyllineTheobromine
The invention relates to the technical field of pharmaceutical preparations, and specifically discloses a pentoxifylline injection and a preparation method thereof. The preparation method is as follows: adding the osmotic pressure regulator in the recipe amount to the water for injection with a total volume of 40-60%, stirring and dissolving, adjusting the pH to 6.5-7.5, then adding the pentoxifylline in the recipe amount, stirring and dissolving, Add water for injection to make up the total amount, adjust the pH to 6.5-7.5, filter through the multi-stage polyethersulfone filter element with decreasing pore size in turn, fill and sterilize to obtain pentoxifylline injection. Compared with the prior art, the present invention achieves the purpose of ensuring product quality stability without adding a stabilizer, and the prescription is simpler. It does not increase, the pH value is stable, the color of the solution is always a colorless and clear solution, and the production cost is also reduced, which is convenient for industrialized production and has high promotion value.
Owner:SHIJIAZHUANG NO 4 PHARMA
A detection method for related substances of 6-chloro-2-hexanone
ActiveCN113466353BEasy to separateEnsure quality stabilityComponent separationPentoxyfyllinePhysical chemistry
The invention relates to the field of analytical chemistry, in particular to a method for detecting related substances of 6-chloro-2-hexanone. The detection method is determined by gas chromatography, and the chromatographic conditions are as follows: the stationary liquid of the capillary chromatographic column: polyethylene glycol or modified polyethylene glycol; The temperature was raised to 220‑240 °C at a rate of 1 min and maintained for 5‑10 min. The detection method provided by the invention has good sensitivity, accuracy, precision, reproducibility, recovery rate and stability, and the detection result is accurate and reliable. The quality stability of the base and the safety of clinical medication are guaranteed.
Owner:沧州临港友谊化工有限公司
Muscular amino acids and nucleosides injection medicinal composition and preparation method thereof
InactiveCN102266351BIncrease breathing vitalityStimulus index increasedPharmaceutical delivery mechanismUnknown materialsDiseaseSodium phosphates
The invention provides a muscular amino acids and nucleosides injection medicinal composition and discloses a preparation method of the medicinal composition. The medicinal composition is prepared from a muscular amino acids and nucleosides injection, pentoxifylline, fructose diphosphate sodium and polyethylene glycol. When the medicinal composition is applied in vitro, the liver homogenate breathing activity of a guinea pig can be increased, an activity stimulation index of breathing activity determined by using a Warburgs respirometer is about 4.2, which is far higher than the stimulation index of the liver homogenate breathing activity of the guinea pig during separate testing by using muscular amino acids and nucleosides, the pentoxifyllinum and the fructose diphosphate sodium, and thus, the treating effect on heart cerebrovascular disease is enhanced greatly.
Owner:长春白求恩制药有限公司
A kind of in vitro fertilization fluid and preparation method thereof
ActiveCN111040991BQuality improvementGood removal effectCulture processCell culture active agentsPhysiologyPentoxyfylline
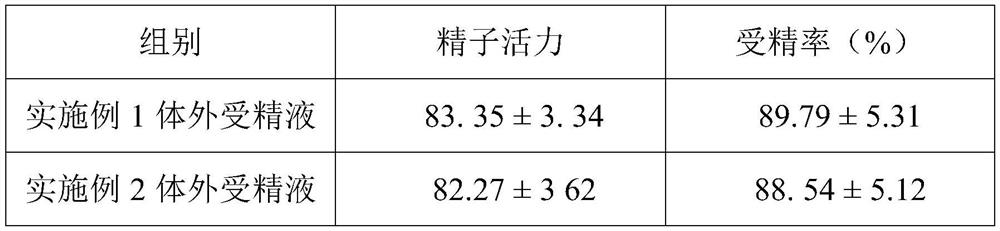
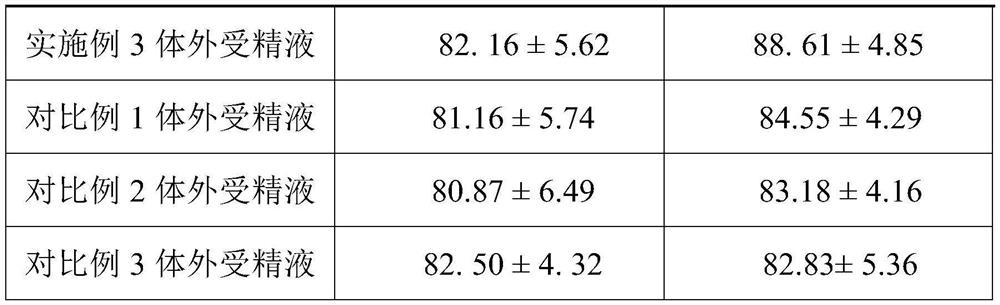
The invention provides an in vitro fertilization liquid and a preparation method thereof, the in vitro fertilization liquid comprises basal fluid, human serum albumin, coenzyme Q10 and pentoxifylline, and the content of coenzyme Q10 in the in vitro fertilization liquid is 0.5- 2mg / L, the content of pentoxifylline in the in vitro fertilization fluid is 0.5-5g / L. The above in vitro fertilization fluid can provide a stable fertilization environment, reduce the damage of active oxygen to sperm and eggs, and can also improve sperm motility and fertilization rate.
Owner:佛山辅康生物科技有限公司
Artificial propagation technology for schizothorax grahami
InactiveCN112931313APromote sexual maturityImprove reproductive performanceClimate change adaptationAnimal feeding stuffAnimal scienceBroodstock
The invention provides an artificial propagation technology for schizothorax grahami, and belongs to the technical field of freshwater fish propagation. The artificial propagation technology comprises the steps of parent fish domestication, parent fish selection, spawning induction, artificial insemination, roe incubation and fry breeding, and the spawning induction adopts pentoxifylline, caffeine and calcium dobesilate as spawning induction assistants; meanwhile a compound feed used in the artificial propagation technology is further provided, the compound feed contains resistant starch which is prepared in an acid environment and has a substitution degree of 0.18-0.21. The artificial propagation technology is stable and controllable in process, low in production cost, high in parent fish domestication success rate, small in production damage, high in fertilization rate and hatching rate, low in fry malformation rate and high in drug utilization rate; and the compound feed has the advantages of excellent swelling degree and slow release property, high digestion conversion rate and high biological effective utilization rate, and can improve the gonad maturation and reproductive capacity of parent fish, improve the quality of seminal fluid and egg granules and reduce the embryonic development malformation rate.
Owner:嘉兴市爵拓科技有限公司
A kind of vasoblocking agent bonded flubororpyrrole derivative and its preparation method and application
ActiveCN111084881BClear structureThe synthesis method is simpleOrganic active ingredientsPhotodynamic therapyTumor therapyPolythylene glycol
The invention discloses a vasoborpyrrole derivative bonded with a blood vessel blocking agent, which is formed by covalently bonding a near-infrared photosensitizer fenpyrrole and a blood vessel blocking agent 2,5-pentoxifylline. Simultaneously, the present invention also discloses a nano-diagnosis reagent obtained by self-assembly of the derivative and the electron-rich amphiphilic polymer methoxy-poly(ethylene glycol)-poly(2-diisopropylamino)methyl methacrylate And its application in type I photodynamic synergistic angioblocking therapy in tumor treatment. The structure of the target product of the present invention is clear, the synthesis process is simple, and the diagnostic and therapeutic reagent based on the target product has both type I photodynamic and pH-responsive vascular blocker release properties. Accurately reach the tumor site, block tumor blood vessels and kill tumor cells, effectively preventing tumor recurrence and metastasis.
Owner:NANJING TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com