Method for detecting related substances of 6-chloro-2-hexanone
A technology related to substances and detection methods, applied in the field of analytical chemistry, to achieve good recovery and stability, good separation, and accurate and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
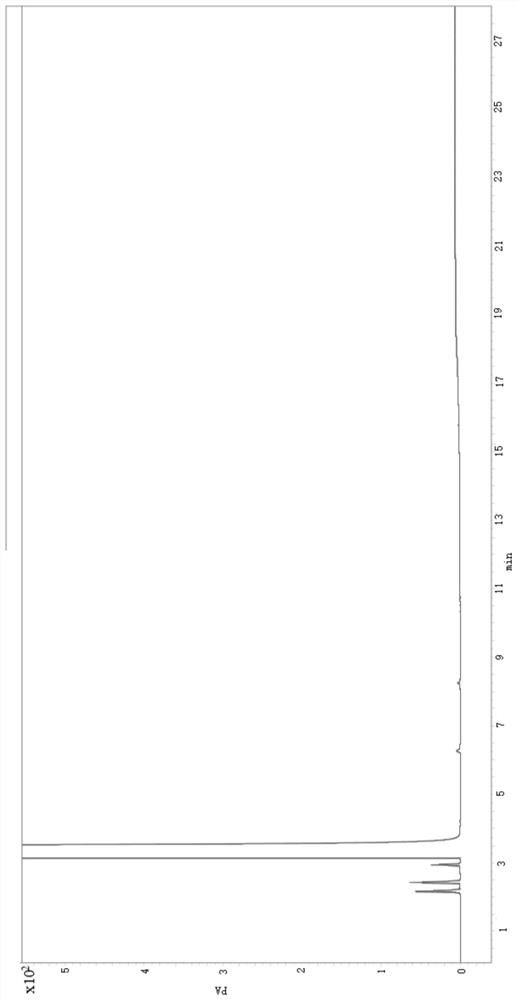
[0032] This embodiment provides a method for detecting related substances of 6-chloro-2-hexanone, the steps are as follows:
[0033] 1.1 Solution preparation
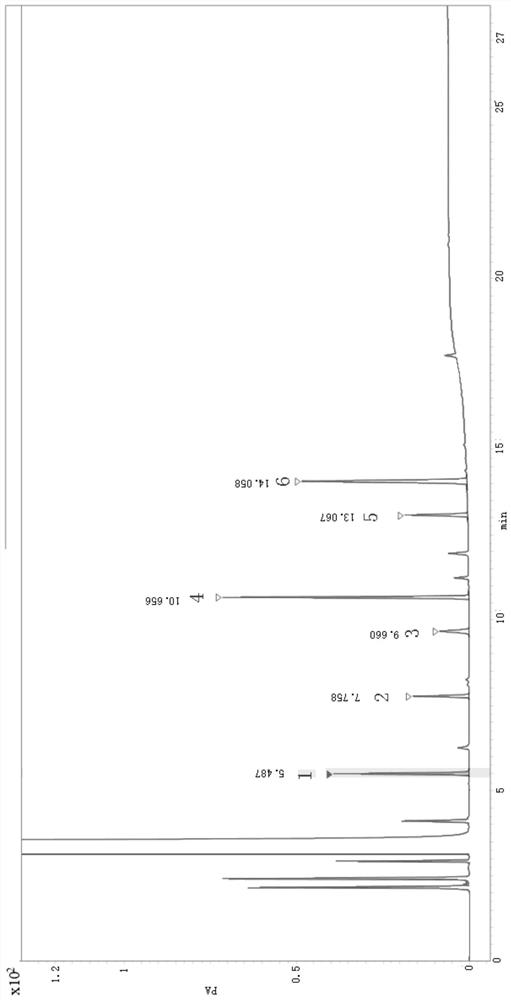
[0034] Preparation of the test solution: Accurately weigh an appropriate amount of 6-chloro-2-hexanone, add dichloromethane to dissolve and dilute to a solution containing 20 mg of 6-chloro-2-hexanone per 1 mL, and obtain the solution.
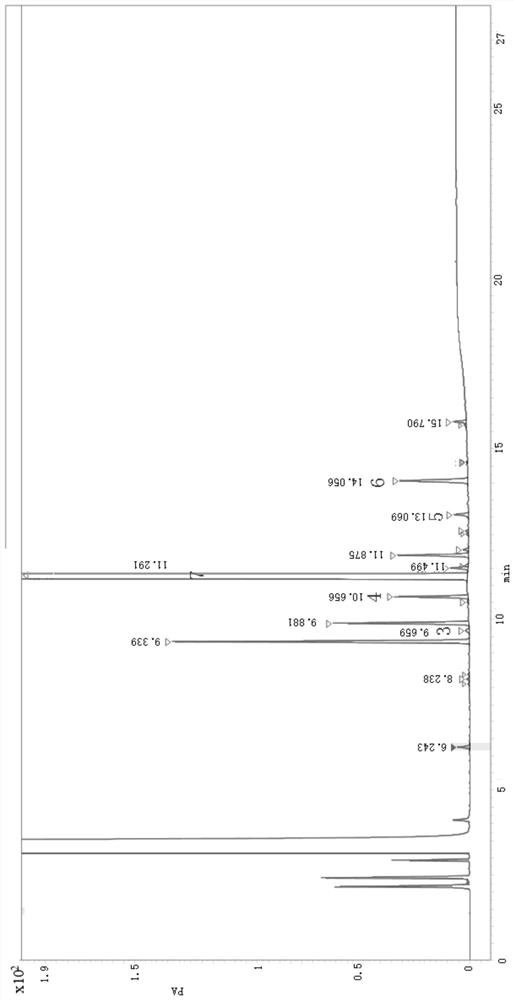
[0035] Preparation of mixed reference solution: Accurately weigh 5-hexen-2-one, bromochloropropane, ethyl acetoacetate, 3-chloro-1-propanol, 5,6-dihydro-3-ethoxycarbonyl -2-Methyl-4H-pyran, 6-caprolactone-2-one appropriate amount of reference substance, respectively dissolved and diluted with dichloromethane, mixed until each 1mL contains about 20μg of 5-hexen-2-one, Bromochloropropane 20 μg, ethyl acetoacetate 20 μg, 3-chloro-1-propanol 80 μg, 5,6-dihydro-3-ethoxycarbonyl-2-methyl-4H-pyran (INT1) 20 μg, 6- A mixed solution of 80 μg of caprolactone-2-one was obtained.
[0036] Prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com