Synthesis method of pentoxifylline
A technology of pentoxifylline and a synthetic method, applied in the direction of organic chemistry and the like, can solve problems such as unreasonable medication, change of indications, etc., and achieve the effects of simple synthesis process, improved reaction yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of synthetic method of pentoxifylline S1
[0027] The present embodiment provides a synthetic method of pentoxifylline S1, the synthetic method is:
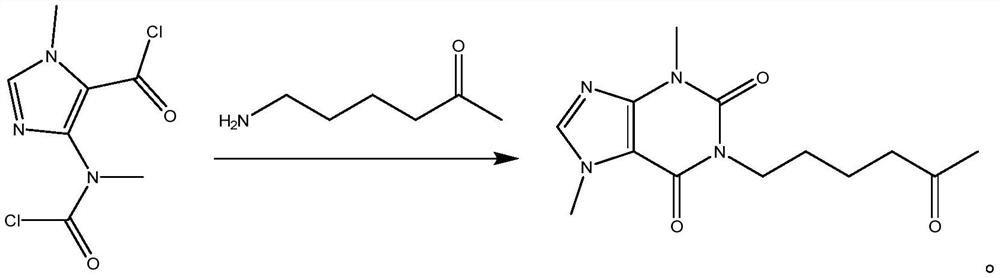
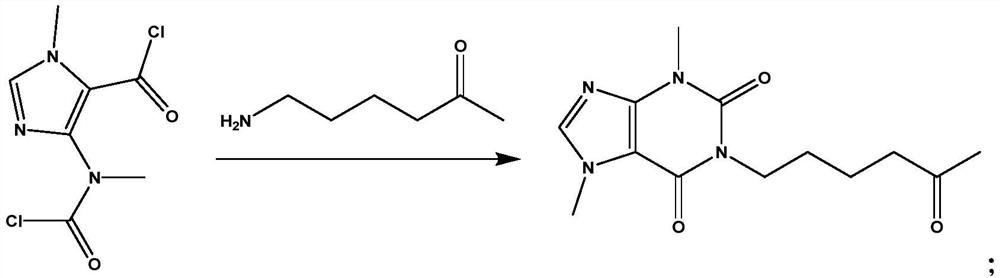
[0028] Measure 70ml of THF into the reaction vessel, start stirring and cool down to -15°C, then weigh 11.5g of amino-5-hexanone into the cooled THF, add 27ml of TEA, mix well, keep warm at -15°C, gradually Add dropwise 23.5g of 4-(nitromethylacyl chloride)-1-methylimidazolium-5-acyl chloride solution dissolved in 20mlTHF, react for 45min, take a small amount of reaction solution as a sample, and inject it into a liquid chromatograph for detection. Amino-5- The absorption peak of hexanone disappears, and the reaction can be terminated, and its reaction formula is:
[0029]
[0030] At -15°C, add 100ml of purified water to the reaction solution and stir for 10min, add 300ml of ethyl acetate and continue to stir for 5min, then let stand to separate layers, remove the water phase, keep the organic phas...
Embodiment 2-4
[0031] The synthetic method of embodiment 2-4 pentoxifylline S2-S4
[0032] The synthesis methods of pentoxifylline S2-S4 provided in Examples 2-4 are basically the same as those in Example 1, the only difference being that some process parameters are different, and the specific process parameters are shown in Table 1.
[0033] Table 1: Process parameter table of pentoxifylline S2-S4
[0034]
[0035]
[0036] Other parameters are all the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com