Blood vessel blocking agent bond joint fluorine-boron pyrrole derivative and preparation method and application thereof
A blood vessel blocking agent, a technology connected with fluborapyr, which is applied in the field of near-infrared dyes and nanomedicine, can solve the problems of photodynamic effect decline and hinder clinical application, etc., and achieve the advantages of simple synthesis method, precise tumor treatment and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
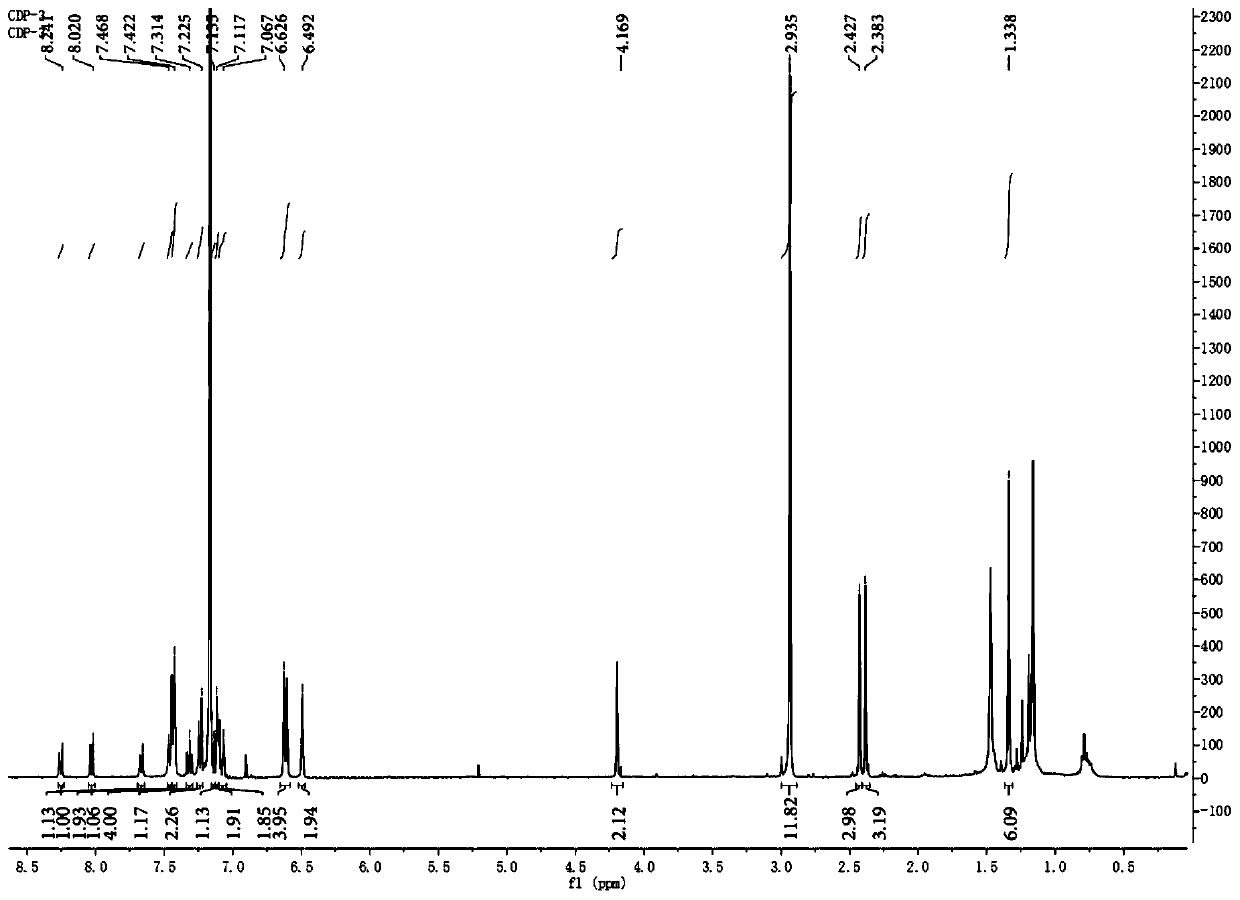
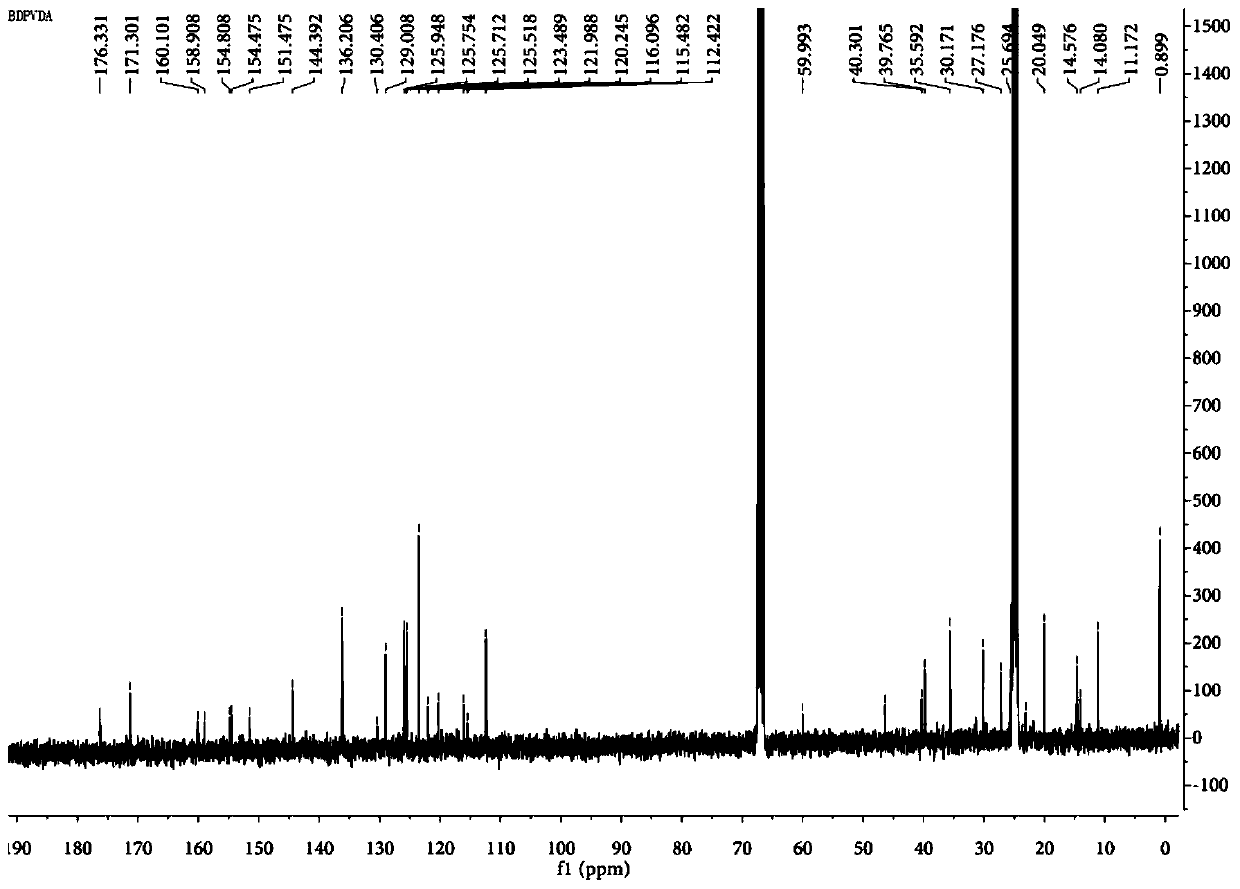
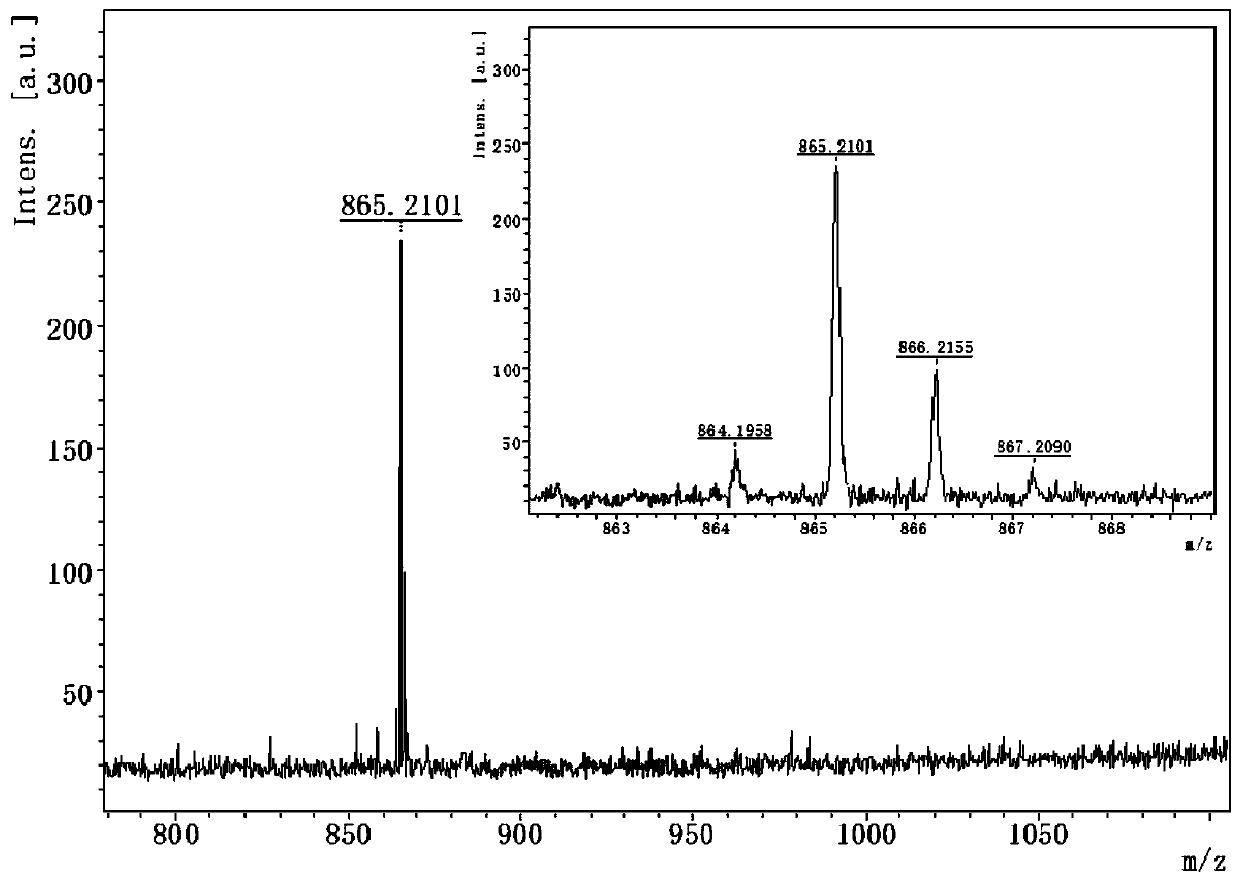
[0055] Vasoblocker-linked BDPVDA synthesis
[0056] (1) In a 500mL flask, dissolve 4-methoxybenzaldehyde (0.37g, 3.0mmol) and 2,4-dimethylpyrrole (0.63g, 6.6mmol) in 90mL tetrahydrofuran, add 0.1mL three Fluoroacetic acid, stirring at room temperature for 12 hours; adding 12 ml of 2,3-dichloro-dicyano-p-benzoquinone (0.68 g, 3.0 mmol) tetrahydrofuran solution to the reaction solution, and continuing the reaction at room temperature for 4 hours; , add 18mL triethylamine and 18mL boron trifluoride diethyl ether dropwise to the reaction liquid, and continue to react for 12 hours; V / V) to obtain an orange solid product (compound 1, 0.459g, about 45% yield), the structural formula is as follows:
[0057]
[0058] The chemical name of compound 1 is: 4-(5,5-difluoro-1,3,7,9-tetramethyl-5H-4λ 4 , 5λ 4 -dipyrrole[1,2-c:2',1'-f][1,3,2]diazaboron)-phenol.
[0059] (2), in a 50mL round bottom flask, dissolve compound 1 (0.34g, 1.0mmol) and 4-dimethylaminobenzaldehyde (0.33g, 2.4mmo...
Embodiment 2
[0067] Preparation of mPEG-PPDA-coated BDPVDA nanoparticles (PBV NPs)
[0068] Dissolve 100mg of mPEG-PPDA (the molecular weight of mPEG is 2000, and the degree of polymerization of PPDA is 80) and 4mg of BDPVDA in 5mL of tetrahydrofuran, stir for 5 minutes, drop it into 80mL of deionized water under ultrasonic conditions (200W), and continue to sonicate for 30 minute. The tetrahydrofuran was removed by vacuum rotary evaporation, and PBV NPs were obtained by filtration with a 220 nm filter head.
[0069] Such as Figure 4 As shown, in the ultraviolet-visible absorption spectrum of PBV NPs, there are strong absorption peaks in the wavelength range of 570nm-850nm, and the highest absorption peak is at 733nm, indicating that PBV NPs have excellent near-infrared absorption properties. At the same time, if Figure 5 As shown, the particle size distribution test showed that the particle size distribution of PBV NPs was 60.7 ± 5.1 nm.
Embodiment 3
[0071] Acid Stimulus-Responsive Release Properties of Vasoblocker DMXAA
[0072] 20 mL of phosphate buffer solution containing PBV NPs (50 μg / mL) was placed in a dialysis bag (molecular weight cut-off: 1000), and placed in phosphate buffer with different pH values (5.0, 6.5, 7.4) for slow release. Record the absorption value of DMXAA at different time points to detect the release amount. Such as Image 6 As shown, the cumulative release rate of DMXAA increased with the decrease of pH value, indicating that PBVNPs have good acid stimulation responsiveness, which will be beneficial to realize the drug release stimulated by tumor micro acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com