Pentoxifylline derivative
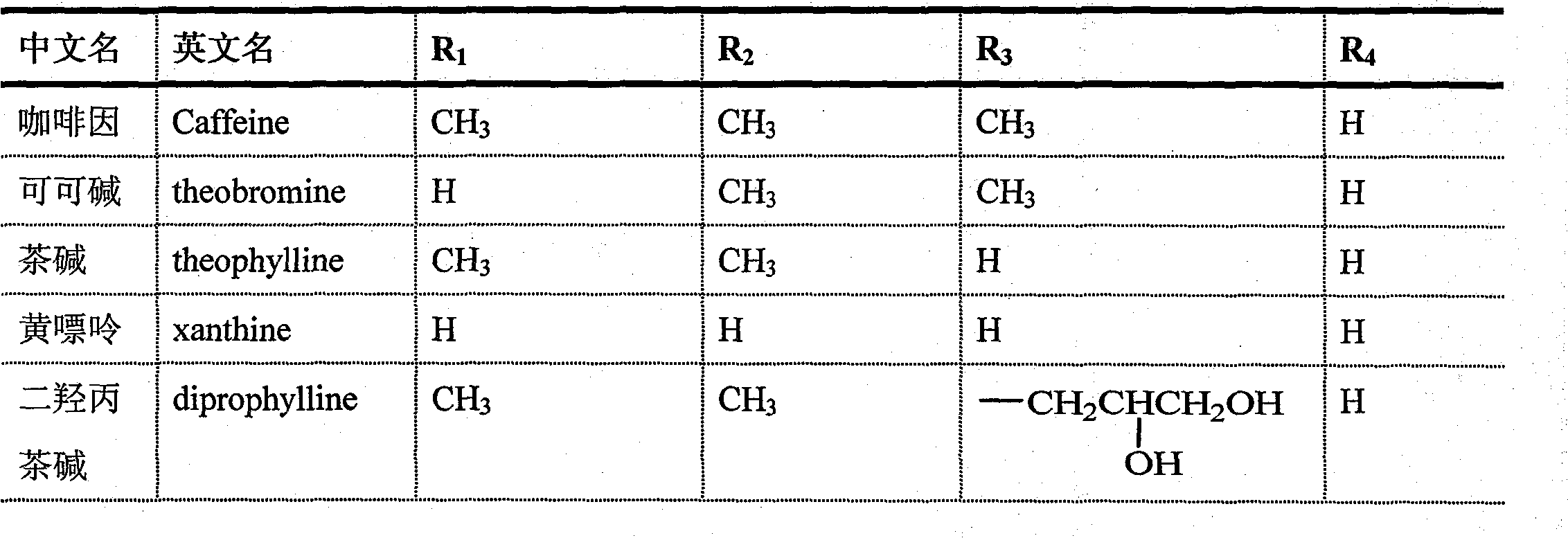
A technology of theobromine derivatives and hexanone, applied in the directions of drug combination, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc., can solve problems such as pain of patients, and achieve the effect of good vasodilation and neuroprotection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0036] Preparation Example 1: Preparation of Intermediate I: (N-Methyl-N-Hydroxyethyl) Aminomethyl Chlorohydrin
[0037] 1. Preparation of N-methylethanolamine
[0038] In a 2000ml four-neck flask, install an electric stirrer, a reflux condenser, a thermometer, and a gas absorption device, add 220.4g (7.097mol) of 25% to 40% methylamine aqueous solution, and stir at 25 to 40°C. Ethylene oxide gas is 58.2g (7.096mol). After ventilation is completed, heat and stir slowly to 120°C, carry out distillation, collect fractions at 120-180°C, and conduct secondary distillation to control the collection of n D 20 The fraction of 1.4385 is 45.6 g of N-methylethanolamine, which is directly reacted in the next step.
[0039] 2, the preparation of (N-methyl-N-hydroxyethyl) aminomethyl chloroethanol
[0040] Add 37g (0.4mol) of epichlorohydrin and 20ml of isopropanol (i-PrOH) into the reaction flask, stir to dissolve, cool down to 18-22°C, and add 37.5g of the compound prepared in the above...
preparation example 2
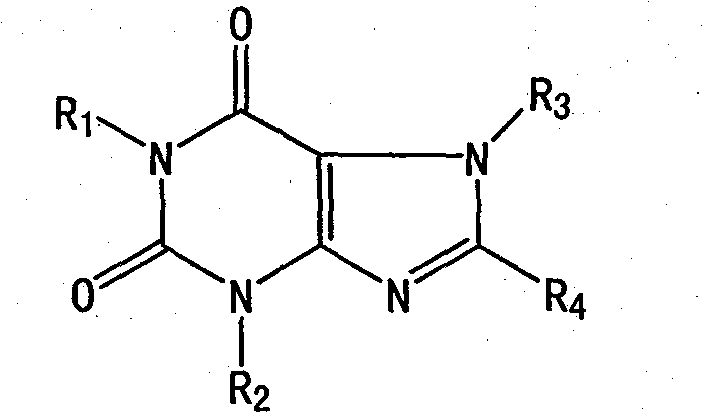
[0041] Preparation Example 2: Preparation of Compound 3-Methyl-7-[2'-Hydroxy-3'-[(2-Hydroxymethyl)methylamino]Propyl]Xanthine
[0042] In the reaction flask, add 125ml of absolute ethanol and 2.86g (0.124) of sodium metal, stir to completely dissolve the metal sodium, then add 20.8g (0.124mol) of powdered 3-methylpurine, heat and reflux for 10 minutes to obtain 3- Sodium salt of methylpurine. Slowly add 20.7 g (0.124 mol) of the isopropanol solution of 1 / 4 (N-methyl-N-hydroxyethyl) aminomethyl chloroethanol in the above preparation example 1 dropwise, stir and reflux after adding, and thin-layer identification At the end of the reaction (developer: ethyl acetate: ethanol: glacial acetic acid = 5:2; 0.1, v:v:v), the reaction was completed in about 4.5 hours. Cool to 0~-5°C, filter, then dissolve the solid with an appropriate amount of 1N NaOH solution, adjust the pH value with 0.5N HCl, control the pH value to 11.5, filter the obtained solid, and use 200ml methanol / chloroform=...
Embodiment 1
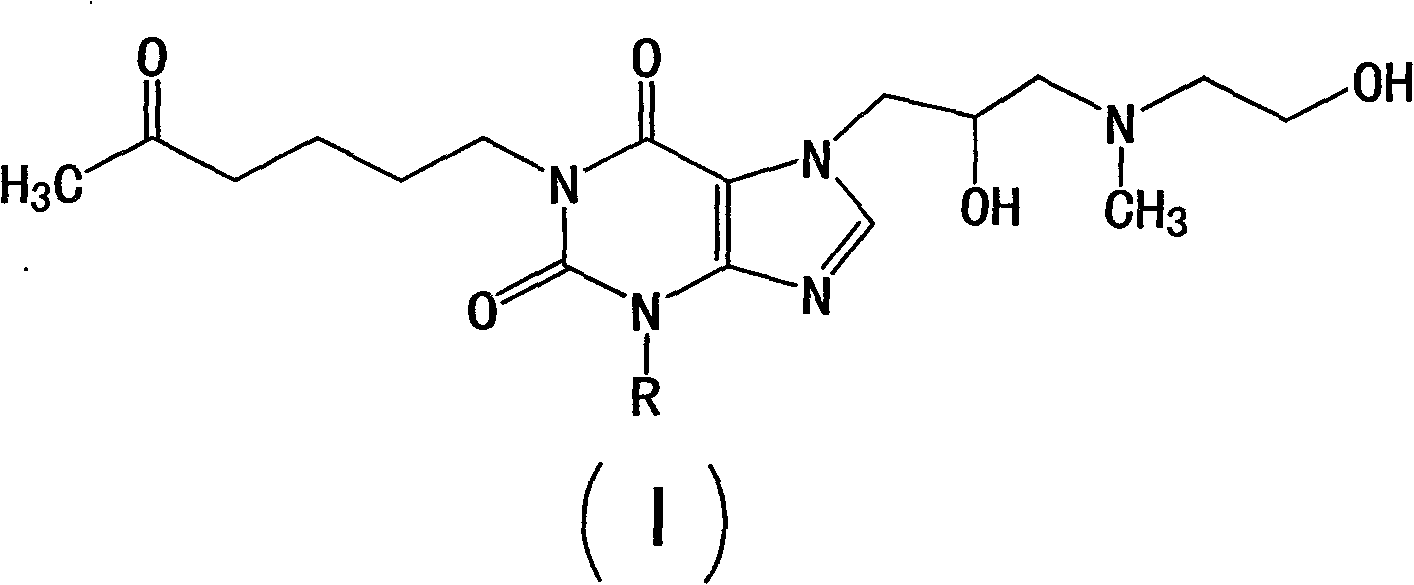
[0044] Example 1: Compound 1-(5'-oxohexyl)-3-methyl-7-[2'-hydroxyl-3'-[(2-hydroxymethyl)methylamino]propyl]xanthine. preparation of
[0045] In a 500ml four-necked flask, install an electric stirrer, a reflux condenser, a thermometer and N 2 To the airway, add 27g (0.09mol) of the compound of Preparation Example 2, 16.8g (0.158mol) of anhydrous potassium carbonate and 250ml DMF in sequence, and add 16g (0.09mol) of 5-oxo-1- Hexyl bromide, after the addition, the mixture was in N 2 Under protection, heat to 120-125°C for 30 minutes, TLC identification of the reaction end point (developing solvent: ethyl acetate: ethanol: glacial acetic acid = 5: 2; 0.1, v: v: v), after the reaction is complete, add 50ml of distilled water, stirred, and then extracted with 200ml×3 chloroform, discarded the aqueous phase, and dried the organic layer with anhydrous sodium sulfate. Filter, concentrate to dryness, and recrystallize the residue with absolute ethanol to obtain 29.5g white crystalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com