Preparation of pH-stable pentoxifylline injection
A technology of theobromine injection and pentoxifylline, which is applied in the field of pH-stable pentoxifylline injection and its preparation, and can solve the problems of increased substance content, large changes in pH value, and poor pH stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
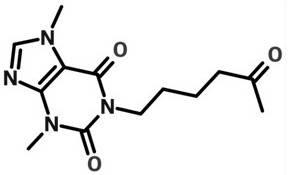
[0030] Per 1000mL Pentoxifylline Injection:
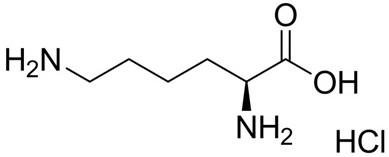
[0031] Pentoxifylline 20g Sodium chloride 6.88g L-Lysine 3g Add water for injection to 1000mL
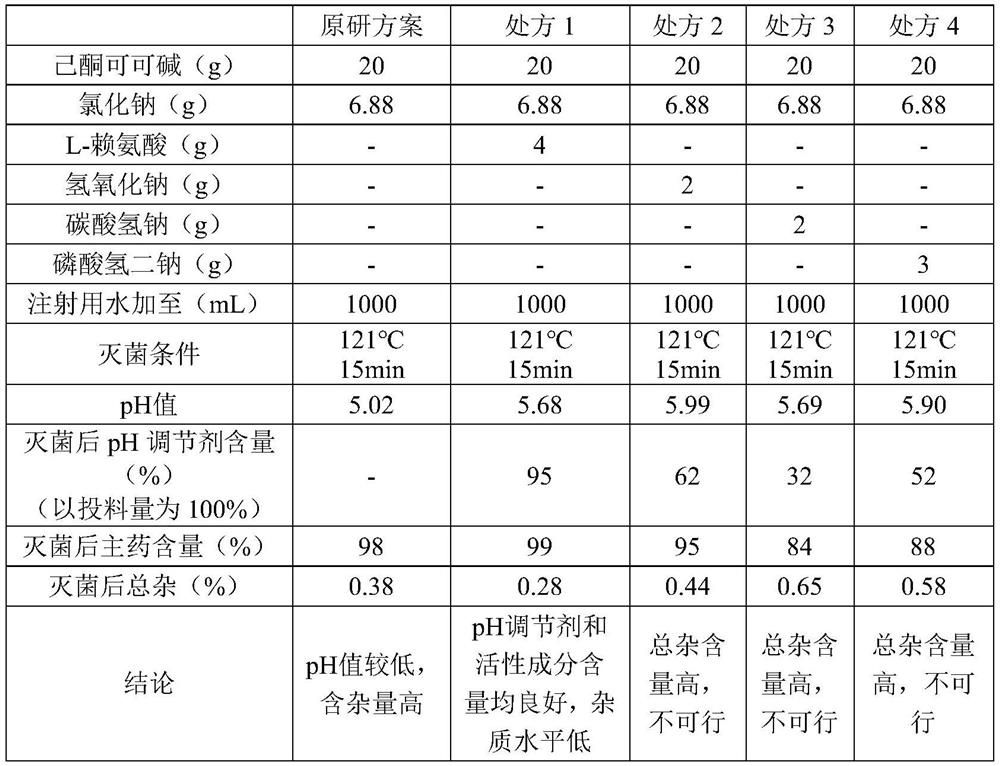
[0032] Add the prescribed amount of sodium chloride, L-lysine, and pentoxifylline to nitrogen-saturated water for injection to dissolve in turn, add water to the full amount, and then pass the liquid medicine through a 0.45 μm coarse filter and a 0.22 μm polyether The sulfone filter core fine filter is filtered into the temporary storage tank, and then the temporary storage tank is filled with a terminal filter, and terminally sterilized at 121°C for 15 minutes to obtain the product.
Embodiment 2
[0034] Per 1000mL Pentoxifylline Injection:
[0035] Pentoxifylline 20g Sodium chloride 6.88g L-Lysine 4g Add water for injection to 1000mL
[0036] Add the prescribed amount of sodium chloride, L-lysine, and pentoxifylline to nitrogen-saturated water for injection to dissolve in turn, add water to the full amount, and then pass the liquid medicine through a 0.45 μm coarse filter and a 0.22 μm polyether The sulfone filter core fine filter is filtered into the temporary storage tank, and then the temporary storage tank is filled with a terminal filter, and terminally sterilized at 121°C for 15 minutes to obtain the product.
Embodiment 3
[0038] Per 1000mL Pentoxifylline Injection:
[0039] Pentoxifylline 20g Sodium chloride 6.88g L-Lysine 5g Add water for injection to 1000mL
[0040] Add the prescribed amount of sodium chloride, L-lysine, and pentoxifylline to nitrogen-saturated water for injection to dissolve in turn, add water to the full amount, and then pass the liquid medicine through a 0.45 μm coarse filter and a 0.22 μm polyether The sulfone filter element fine filter is filtered into the temporary storage tank, and then the temporary storage tank is filled with a terminal filter, and terminally sterilized at 121°C for 15 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com