Pentoxifylline as well as synthesis method and application thereof
A technology of pentoxifylline and a synthesis method, which is applied in the field of medicine, can solve the problems of dosage limitation, great personal harm and the like, and achieves the effects of simple synthesis process, few synthesis steps and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
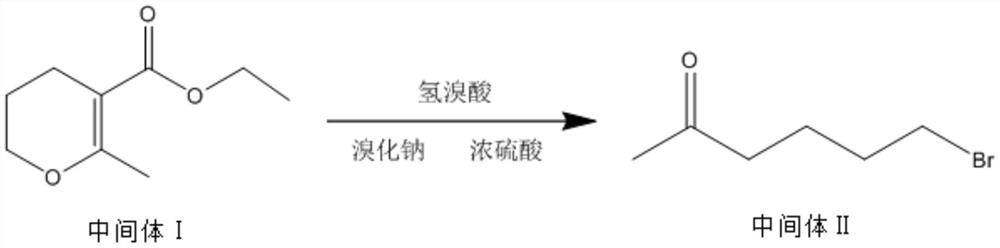
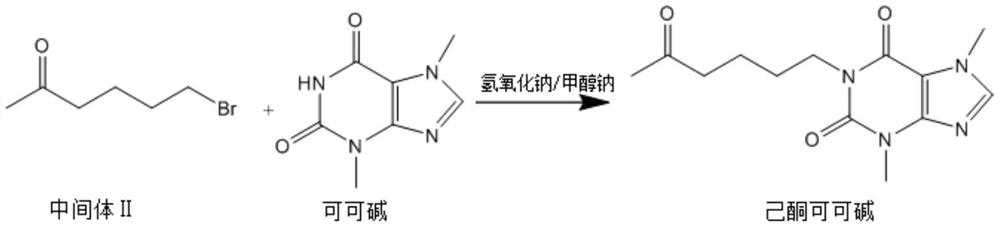
[0038] The invention discloses a synthetic method of pentoxifylline, comprising the following steps:
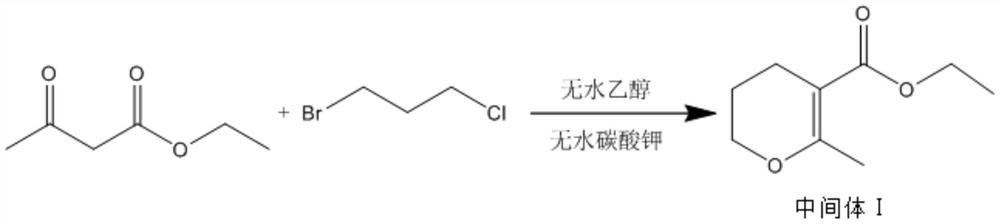
[0039] Step 1: Synthesis of Intermediate Ⅰ
[0040] Add 6.5-7.5 parts of absolute ethanol, 2-3 parts of anhydrous potassium carbonate and 1-2 parts of ethyl acetoacetate into the reaction vessel, use absolute ethanol and anhydrous sodium carbonate, avoid the introduction of water, stir evenly, Then add 0.5-1.5 parts of 1,3-bromochloropropane, heat up to reflux reaction, the temperature is 70-80°C, the time is 7-8 hours, and the reaction product is separated and purified to obtain intermediate I;
[0041] Concrete reaction process is as follows:
[0042]
[0043] The specific steps of separation and purification are:
[0044] Cool the reaction product to room temperature (20°C ~ 30°C), collect the filtrate after centrifugation, in order to collect more fully, add absolute ethanol to the filter cake after the first centrifugation, stir and beat, reflux for 20 ~ 40min, and ...
Embodiment 1
[0069] Step 1: Synthesis of Intermediate Ⅰ
[0070] (1) Feeding 1: Turn on the vacuum pump, add 6.5 parts of absolute ethanol to the 1500L glass-lined reactor; open the solid feeding port and add 2 parts of anhydrous potassium carbonate (crushed through a 100-mesh sieve), and stir;
[0071] (2) Feeding 2: Add 1 part of ethyl acetoacetate at 20°C, after the addition is complete, stir at the temperature for 1 hour;
[0072] (3) Reaction: add 0.5 parts of 1,3-bromochloropropane, after the addition is complete, steam through the jacket, heat up to 70°C, and reflux for 7 hours;
[0073] (4) Separation and washing: Cooling water is passed through the jacket, the temperature is lowered to 20°C, centrifuged, the filter cake is stirred and beaten with 4 parts of absolute ethanol and refluxed for 30 minutes, and centrifuged;
[0074] (5) Concentration 1: Combine and collect the filtrate into a 5000L glass-lined glass-lined reaction kettle, pass steam, and concentrate under reduced pres...
Embodiment 2
[0105] Step 1: Synthesis of Intermediate Ⅰ
[0106] (1) Feeding 1: Turn on the vacuum pump, add 7.5 parts of absolute ethanol to the 1500L glass-lined reactor; open the solid feeding port and add 3 parts of anhydrous potassium carbonate (crushed through a 100-mesh sieve), and stir;
[0107] (2) Feeding 2: Add 2 parts of ethyl acetoacetate at 30°C, after the addition is complete, stir at the temperature for 2 hours;
[0108] (3) Reaction: Add 1.5 parts of 1,3-bromochloropropane, after the addition is complete, steam through the jacket, heat up to 80°C, and reflux for 8 hours;
[0109] (4) Separation and washing: Cooling water is passed through the jacket, the temperature is lowered to 30°C, centrifuged, the filter cake is stirred and beaten with 4.5 parts of absolute ethanol and refluxed for 40 minutes, and centrifuged;
[0110] (5) Concentration 1: Combine and collect the filtrate into a 5000L glass-lined glass-lined reaction kettle, pass steam, and concentrate under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com