Method for detecting genotoxic impurity in pentoxifylline
A technology of pentoxifylline and genotoxicity, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem that the sensitivity cannot meet the detection requirements, and achieve the effect of improving sample analysis efficiency, strong specificity, and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This embodiment provides a detection method for genotoxic impurities in pentoxifylline, the detection method comprising the following steps:
[0055] Step 1, preparing the test solution, reference solution, blank solution and mixed solution;
[0056] The preparation of above-mentioned reference substance solution: get 3-(3-chloropropoxyl)-2-enoic acid ethyl ester reference substance, be mixed with methanol the reference substance stock solution that concentration is 499.37ng / mL, then to above-mentioned reference substance storage solution was diluted to obtain a concentration of 12.5ng / mL reference substance solution;
[0057] The preparation of above-mentioned need testing solution: take need testing 100mg, accurately weigh, place 10mL volumetric flask, add methanol to dissolve and dilute to scale, as need testing solution;
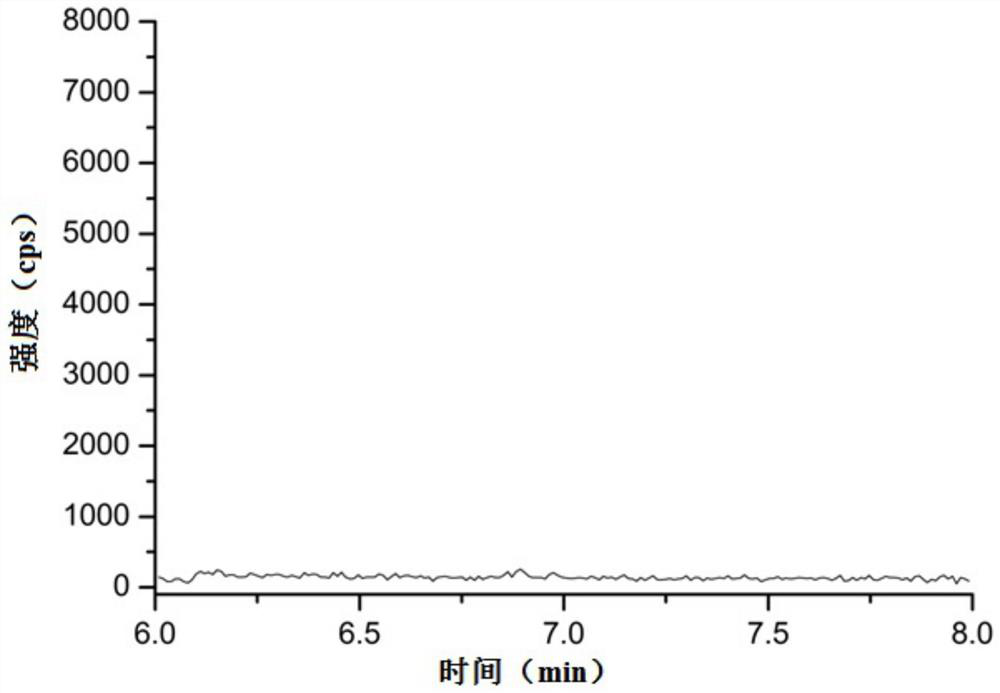
[0058] Above-mentioned blank solution is methanol solvent;
[0059] Preparation of the above mixed solution: Take 100 mg of the test product and ...
Embodiment 2
[0072] Embodiment 2 detection limit and quantitative limit
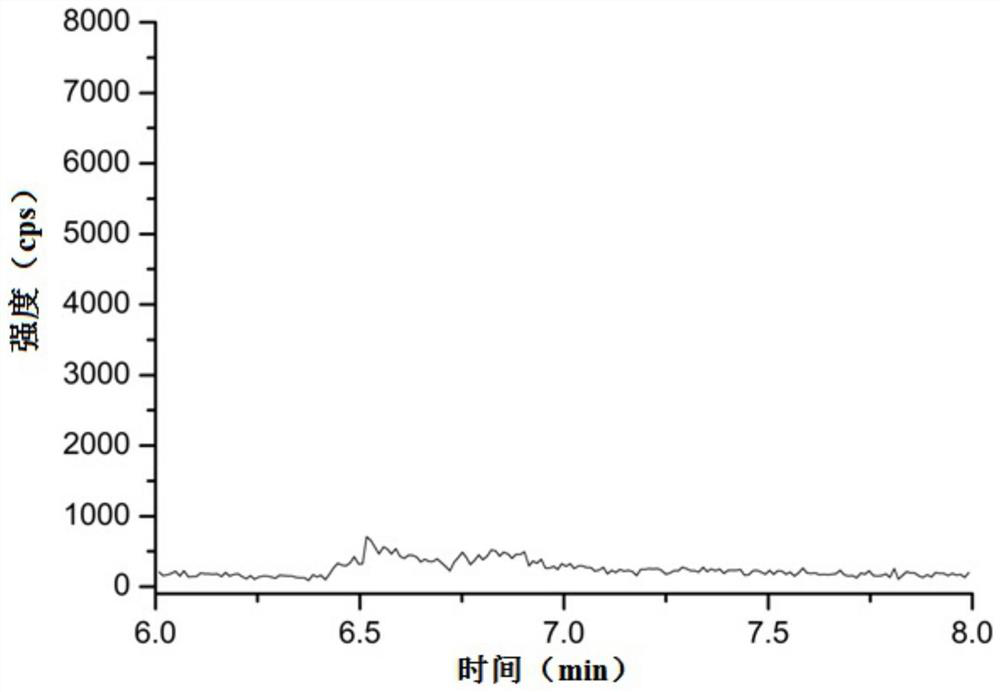
[0073] Limit of detection: the reference substance solution with a concentration of 12.5 ng / mL prepared in Example 1 was quantitatively diluted step by step with methanol, and then detected by liquid chromatography-mass spectrometry. The specific conditions of liquid chromatography and mass spectrometry are as in Example 1 As mentioned above, record the spectrogram, and obtain the detection limit according to the signal-to-noise ratio not lower than 3:1, and the results are shown in Table 1.
[0074] Quantitation limit: the concentration prepared in Example 1 is that the 12.5ng / mL reference substance solution is quantitatively diluted step by step with methanol, and then the liquid chromatography-mass spectrometry method is used to detect the quantitative limit solution. The specific conditions of liquid chromatography and mass spectrometry are as follows: As described in Example 1, the spectrogram was recorded, and ...
Embodiment 3
[0080] Embodiment 3 linear relationship
[0081] The reference substance stock solution with a concentration of 499.37ng / mL prepared in Example 1 was diluted with methanol to obtain concentrations of 2.50ng / mL, 4.99ng / mL, 9.99ng / mL, 12.48ng / mL, and 19.97ng / mL respectively. , 24.97ng / mL linear solution.
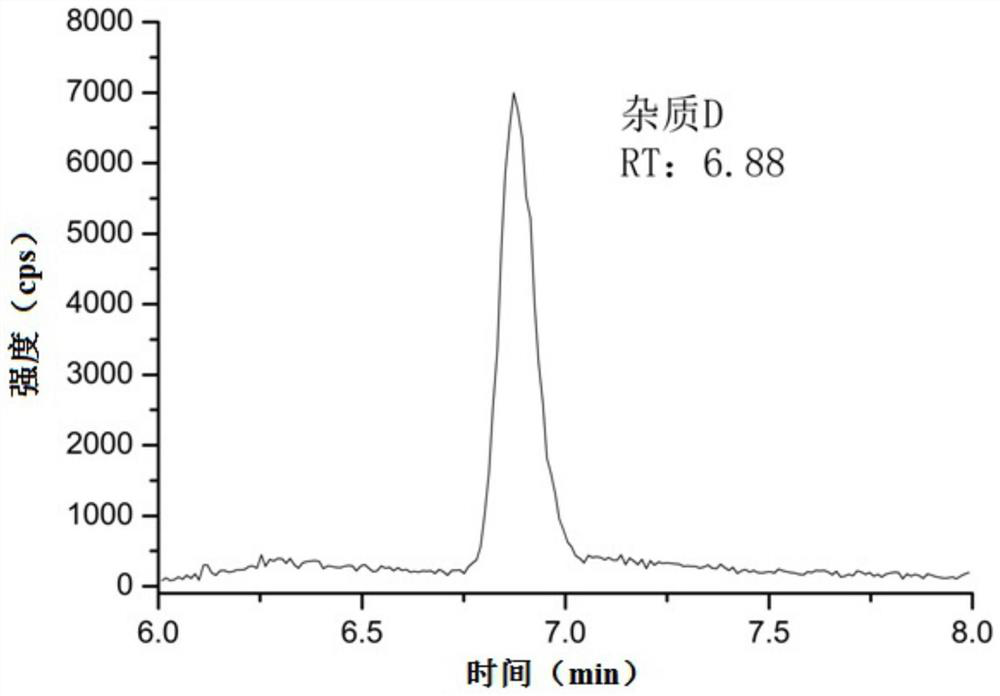
[0082] The linear solution prepared above was detected by liquid chromatography-mass spectrometry. The specific conditions of liquid chromatography and mass spectrometry were as described in Example 1, and the spectra were recorded. Taking the concentration (ng / mL) of 3-(3-chloropropoxy)-2-enoic acid ethyl ester (impurity D) as the abscissa, taking the peak area as the ordinate, draw a standard curve, and calculate the regression equation, the result See Table 3, see the linear graph Figure 5 . It can be seen from the results that the impurity D has a good linear relationship within the concentration range of 2.50ng / mL-24.97ng / mL.
[0083] Table 3 impurity D linear relati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com