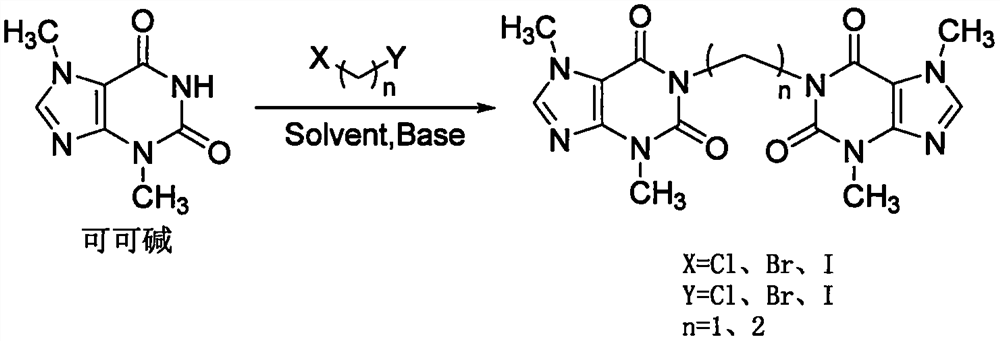
Preparation method of pentoxifylline impurity
A technology of pentoxifylline and theobromine, applied in the direction of organic chemistry and the like, can solve the problems of high price, long supply cycle, failure to find the preparation method of impurity E and impurity K, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
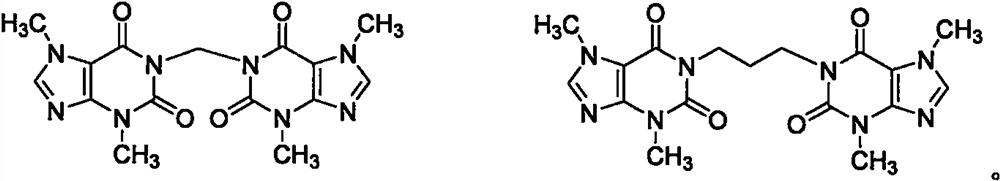
[0024] At room temperature, add 50mL of N,N-dimethylformamide into a 100mL three-necked flask, start stirring, add 9.0g of theobromine, 10.6g of sodium carbonate, raise the temperature to 70°C, add 4.5g of dibromomethane dropwise, complete the addition, and keep warm Continue to stir at 70°C for 4h, stop the reaction, cool, filter, pour the residue into 200mL water, stir, add 2N sodium hydroxide to adjust the pH to 10-11, filter, filter out insoluble matter, add 100mL ethyl acetate to the filtrate to extract, organic Separate the phases, add 50 mL of water to wash, add 5.0 g of anhydrous sodium sulfate to the organic phase, dry for 30 min, filter, and evaporate the organic phase to dryness to obtain a white solid, which is purified by column chromatography to obtain 7.0 g of a white solid, with a yield of 75.3%. .
[0025] 1 H-NMR (500MHz, DMSO-D6): δ7.86(s, 2H), 5.02(s, 2H), 3.68(s, 6H), 3.32(s, 6H); HRMS: m / z=373.1295[M +1] + .
Embodiment 2
[0027] At room temperature, add 50 mL of N,N-dimethylacetamide into a 100 mL three-necked flask, start stirring, add 9.0 g of theobromine, 10.4 g of potassium carbonate, raise the temperature to 80°C, add 7.0 g of diiodomethane dropwise, and complete the addition. Insulate at 80°C and continue to stir for 2 h, stop the reaction, cool, filter, pour the residue into 200 mL of water, stir, add 2N sodium hydroxide to adjust the pH to 10-11, filter, filter out insoluble matter, add 100 mL of ethyl acetate to the filtrate for extraction, The organic phase was liquid-separated, washed with 50 mL of water, the organic phase was dried by adding 5.0 g of anhydrous sodium sulfate for 30 min, filtered, and the organic phase was evaporated to dryness to obtain a white solid, which was subjected to column chromatography (ethyl acetate:n-hexane=1:5 ~1:3, v / v) to obtain 7.5 g of white solid with a yield of 80.6%.
Embodiment 3
[0029] At room temperature, add 40 mL of tetrahydrofuran and 10 mL of water into a 100 mL three-necked flask, start stirring, add 9.0 g of theobromine, 2.2 g of sodium hydroxide, and dropwise add 7.0 g of diiodomethane. Cool, filter, pour the residue into 200mL water, stir, add 2N sodium hydroxide to adjust the pH to 10-11, filter, filter out insoluble matter, add 100mL dichloromethane to the filtrate for extraction, separate the organic phase, add 50mL water to wash, The organic phase was dried by adding 5.0 g of anhydrous sodium sulfate for 30 min, filtered, and the organic phase was evaporated to dryness to obtain a white solid, which was purified by column chromatography to obtain 6.8 g of a white solid with a yield of 73.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com