Nano-carrier sustained release preparation for treating cardiovascular diseases and preparation method of nano-carrier sustained release preparation
A nano-carrier and sustained-release preparation technology, which is applied in the field of nano-carrier sustained-release preparations for the treatment of cardiovascular diseases and its preparation, can solve the problems of affecting the curative effect and difficult storage of pentoxifylline, and achieve the reduction of adverse reactions, stable dissolution rate, The effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
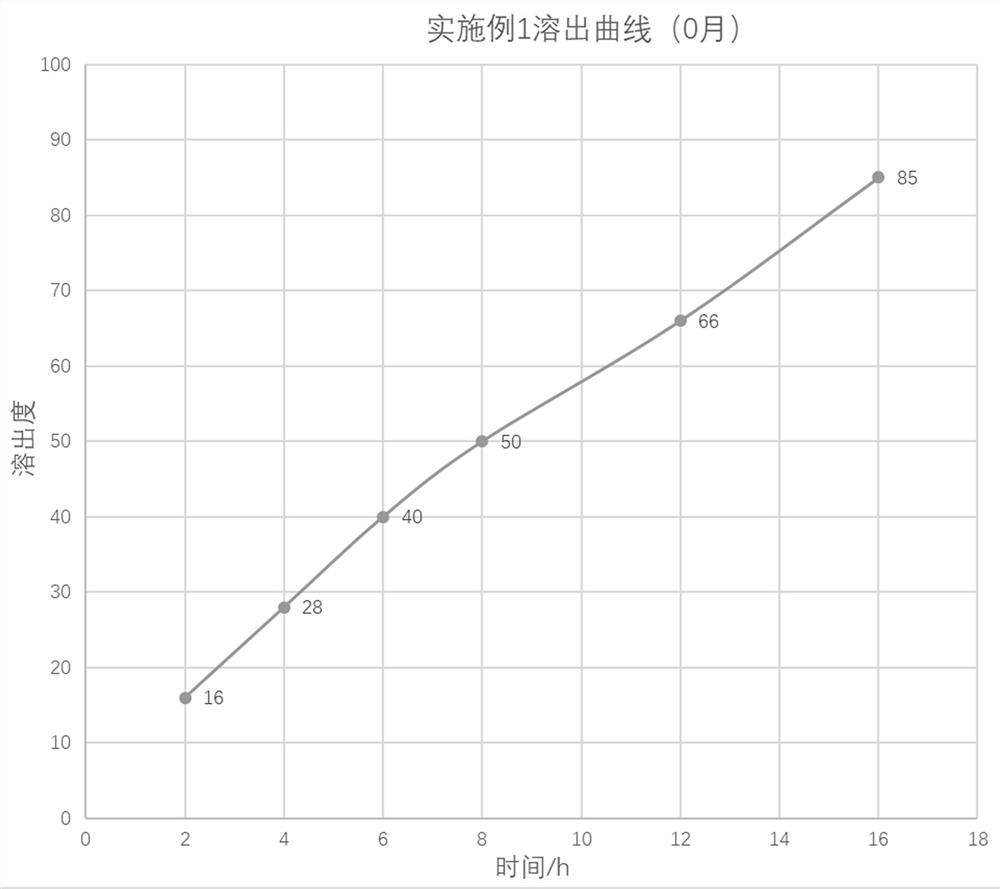
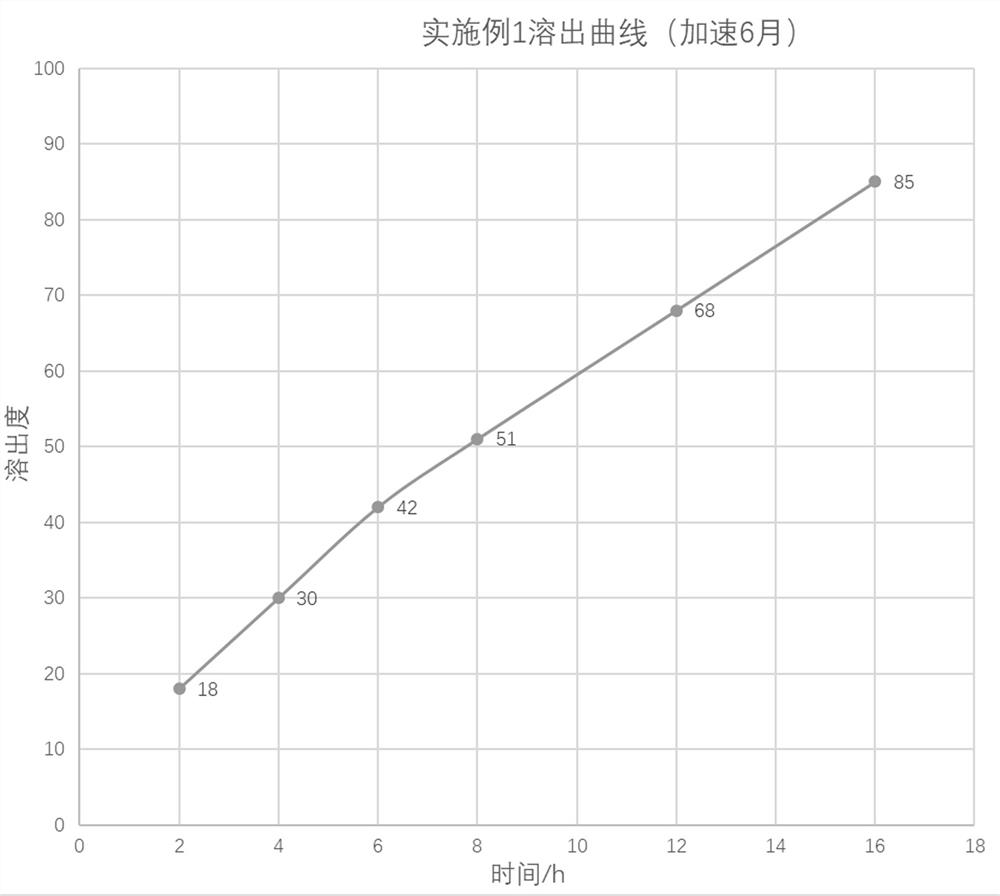
preparation example 1
[0040] Preparation Example 1: Preparation of Pentoxifylline Nano-carrier Freeze-dried Powder
[0041] The molecular weight of chitosan oligosaccharide is 2400Da; the weight ratio of chitosan oligosaccharide, deoxycholic acid and albumin is 1: 2.8: 3.2; the specific preparation method is as follows:
[0042] (1) Preparation of oligochitosan-deoxycholic acid composite microspheres
[0043] The chitosan solution with a concentration of 1 mg / mL is adjusted to a pH value of 5.5 with sodium hydroxide solution to obtain a chitosan solution for subsequent use;
[0044] The deoxycholic acid solution with a concentration of 1 mg / mL was adjusted to pH 5.5 with dilute hydrochloric acid solution; the deoxycholic acid solution was obtained and set aside.
[0045] The chitosan solution and the deoxycholic acid solution were uniformly mixed according to a volume ratio of 1:2.8, and stirred at 25° C. for 2 h to form a solution of chitosan-deoxycholic acid composite microspheres.
[0046] (2)...
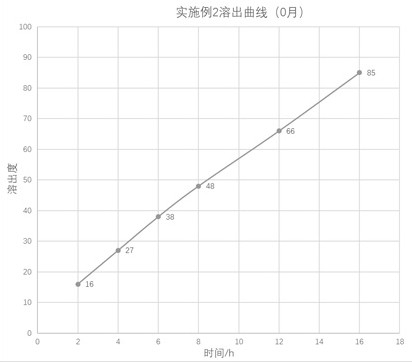
preparation example 2
[0051] Preparation example 2: Preparation of pentoxifylline nanocarrier lyophilized powder
[0052] Chitooligosaccharide molecular weight is 2300Da, all the other are the same as preparation example 1.
preparation example 3
[0053] Preparation example 3: Preparation of pentoxifylline nanocarrier lyophilized powder
[0054] Chitooligosaccharide molecular weight is 2500Da, all the other are the same as preparation example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com