Preparation method and application of hemoporphyrin/verapamil conjugates
A technology of methyl verapamil and hematoporphyrin, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of limited application and achieve the goal of improving physical and chemical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
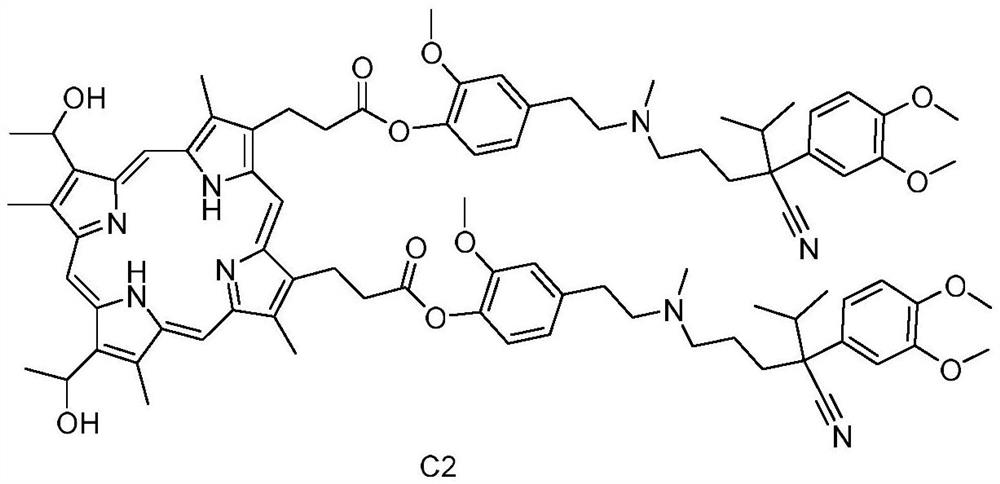
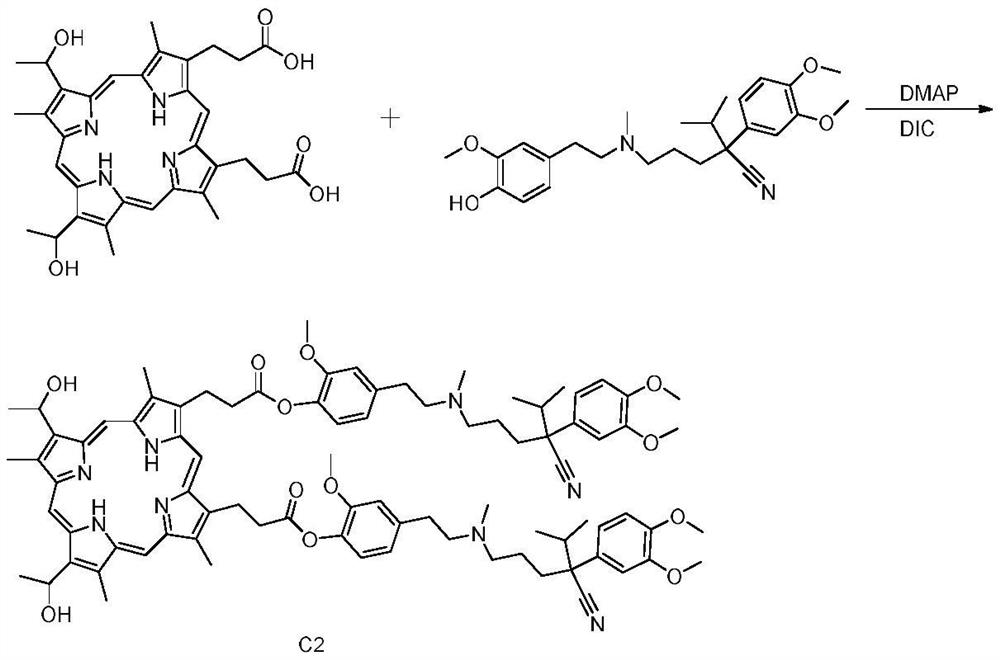
[0030] Synthesis of Hematoporphyrin / Verapamil Conjugate C2:
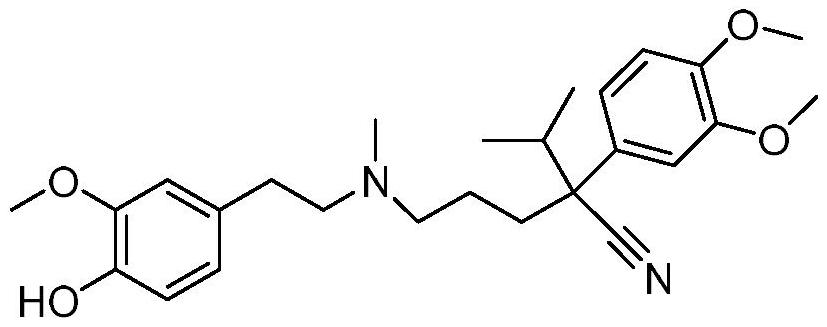
[0031] Dissolve 0.5mmol hematoporphyrin in a reaction vessel containing 50mL N,N′-diisopropylcarbodiimide (DIC), stir the reaction vessel on a magnetic stirrer, and then add 0.5mmol p-O - Desmethyl verapamil and 5 mmol 4-dimethylaminopyridine (DMAP), stirred at room temperature for 12 hours, and the progress of the reaction was detected by thin-plate chromatography (TLC).
[0032] After the reaction was completed, water was added for washing. After drying, the solvent was evaporated to obtain a crude reaction product. The crude product was dissolved in ethyl acetate, and silica gel equivalent to three times the mass of the crude product was added. The dark brown compound C2 was obtained by eluting with dichloromethane:methanol volume ratio 40:1 in silica gel column chromatography with a yield of 21%.
[0033] The results of the hydrogen spectrum and mass spectrum of the hematoporphyrin / verapamil conjugate C2 prep...
Embodiment 2
[0037] Activity Evaluation of Hematoporphyrin / Verapamil Conjugate C2
[0038] 1. Experimental equipment and materials: instrument ultra-clean bench (Sujing Group Antai Company), constant temperature incubator (Thermoelectron Corporation), microplate reader (BIO-RAD Company), inverted biological microscope (Chongqing Optical Instrument Factory); reagents: Cell culture medium RPMI-1640, DMEM (high glucose) (GIBCO company), fetal bovine serum (Hangzhou Sijiqing Co., Ltd.), tetramethylazolazolium blue (MTT) (Sigma company product), DMSO (Sigma company); Cell lines: human liver cancer cell lines HepG-2 and Bel-7402, human breast cancer cell line MCF-7, human prostate cancer cell line PC-3, and human colon cancer cell line HCT-116.
[0039] 2. Experimental method
[0040] 2.1 Cell inhibitory activity test method:
[0041] The above cells were stored at 37°C, 5% CO 2 Routine cultivation in a saturated humidity incubator. The culture medium is RPMI1640 cell culture medium containi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com