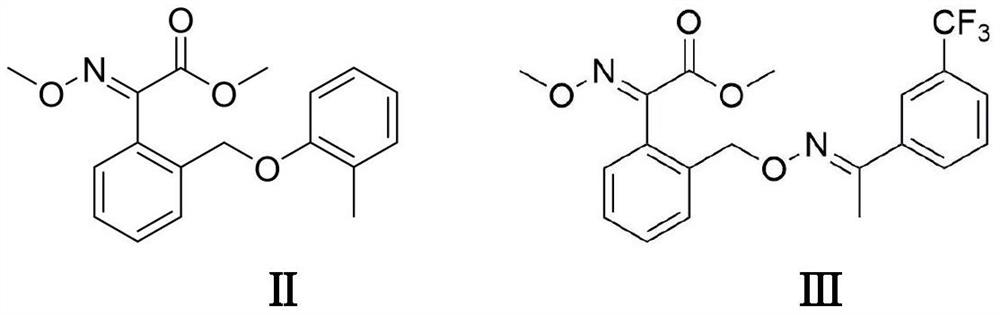
Electrooxidative Preparation Method of Kresstrobin and Trifloxystrobin Intermediates
A technology of electro-oxidation and electrolytic cell, which is applied in the direction of oxime preparation, electrolytic components, electrolytic process, etc., can solve the problems of complex reaction process and harsh conditions, and achieve the effect of simplifying the process and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
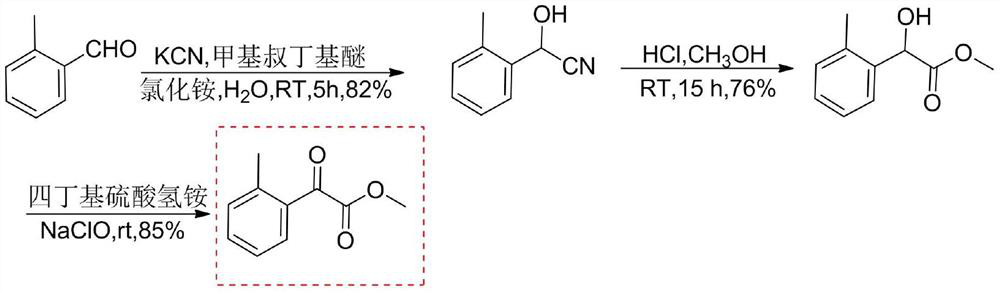
[0050] Electrooxidative Preparation of Methyl 2-Methylacetophenone
[0051]
[0052] Install the anode in the electrolyzer (carbon felt: 10×10×2mm 3 ) and cathode (platinum mesh: 10×10×2mm 3 ); Add a magnetic stir bar in the electrolytic cell, 82mg (0.5mmol) methyl 2-methylphenylacetate, 163mg (1.0mmol) N-hydroxyphthalimide and 342mg (1mmol) tetrabutyl Add 10 mL of acetonitrile and 2 mL of water to dissolve ammonium chlorate, add 80 mg (1 mmol) of pyridine, electrolyze with a constant current of 10 mA at 20 °C for 8 h, spin evaporate the reaction solution, dissolve in ethyl acetate, wash with water three times, and dry the organic layer with anhydrous sodium sulfate , rotary steamed to obtain 76mg methyl 2-methylacetophenone; yield 85%; 1 HNMR (400MHz, CDCl 3 )δ: 7.71~7.28 (m, 4H, benzene ring), 3.96 (s, 3H, OCH 3 ), 2.61 (s, 3H, CH 3 ).
Embodiment 2
[0054] Electrooxidative Preparation of Ethyl 2-Methylacetophenone
[0055]
[0056] Install the anode in the electrolyzer (carbon felt: 10×10×2mm 3 ) and cathode (platinum mesh: 10×10×2mm 3); Add a magnetic stirrer in the electrolytic cell, 89mg (0.5mmol) ethyl 2-methylphenylacetate, 82mg (0.5mmol) N-hydroxyphthalimide and 342mg (1mmol) tetrabutyl Add 10 mL of acetonitrile and 2 mL of water to dissolve ammonium perchlorate, add 80 mg (1 mmol) of pyridine, electrolyze with a constant current of 10 mA at 50 ° C for 3 h, spin the reaction solution, dissolve in ethyl acetate, wash with water three times, and dry the organic layer with anhydrous sodium sulfate , rotary steamed to obtain 84mg methyl 2-methylacetophenone; yield 88%; 1 H NMR (400MHz, CDCl 3 )δ: 7.73–7.30 (m, 4H, benzene ring), 4.47 (q, J=7.1Hz, 2H, OCH 2 ), 2.64 (s, 3H, CH 3 ), 1.45(t, J=7.1Hz, 3H, CH 3 ).
Embodiment 3
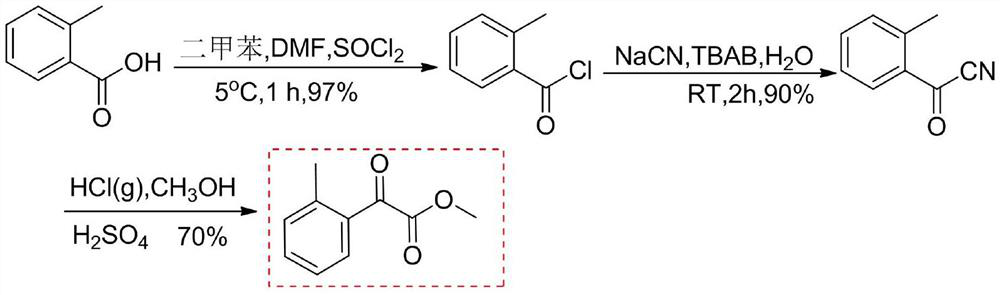
[0058] Electrooxidative Preparation of Methyl 2-Methylacetophenone
[0059]
[0060] The anode of the electrolytic cell is carbon felt (10×10×2mm 3 ), the cathode is platinum mesh (10×10×2mm 3 ), a magnetic stirrer was added to the tank; 0.5mmol (0.082g) methyl 2-methylphenylacetate, 1.0mmol (0.163g) N-hydroxyphthalimide 0.15mmol 2,6-dimethyl Pyridine and 1mmol (0.342g) tetrabutylammonium perchlorate were added to the electrolytic cell, 9.5mL acetonitrile and 0.5mL water were dissolved, electrolyzed at a constant current of 5mA under an oxygen atmosphere at 60°C, and the reaction was stopped when the cell voltage reached 3.0V, and the reaction solution After vacuum distillation and drying, ethyl acetate was dissolved, washed three times with water, and the organic layer was dried over anhydrous sodium sulfate to obtain 0.069 g of methyl 2-methylacetophenone; the yield was 78%; 1 H NMR (400MHz, CDCl 3 )δ: 7.71–7.65 (m, 1H, benzene ring), 7.49 (d, J=5.3Hz, 1H, benzene ring...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com