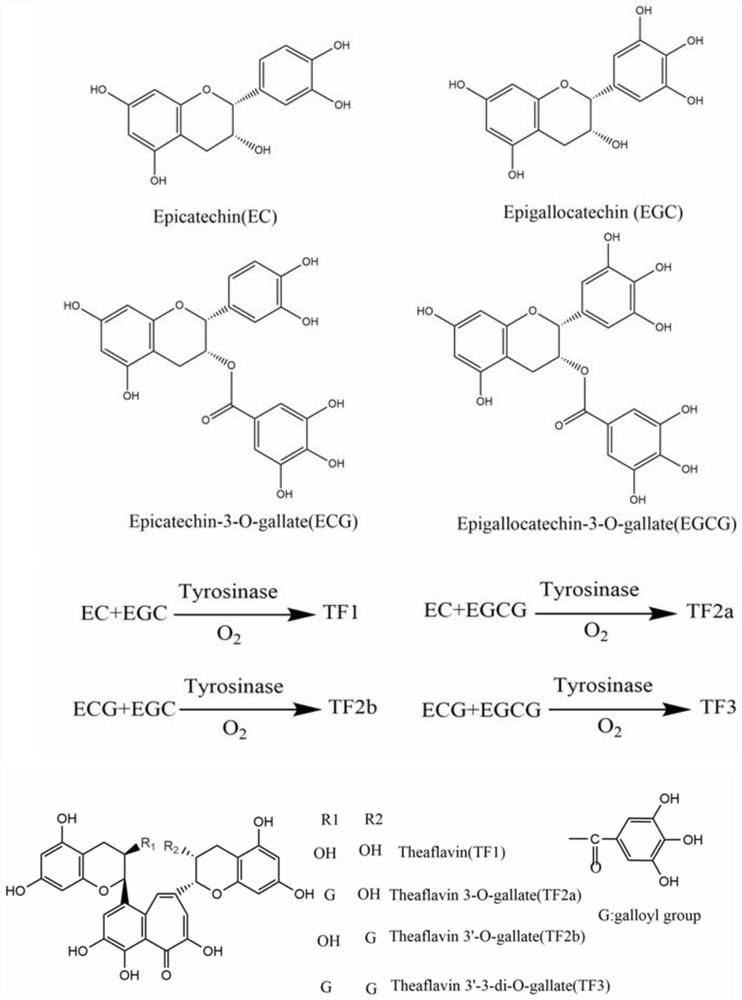
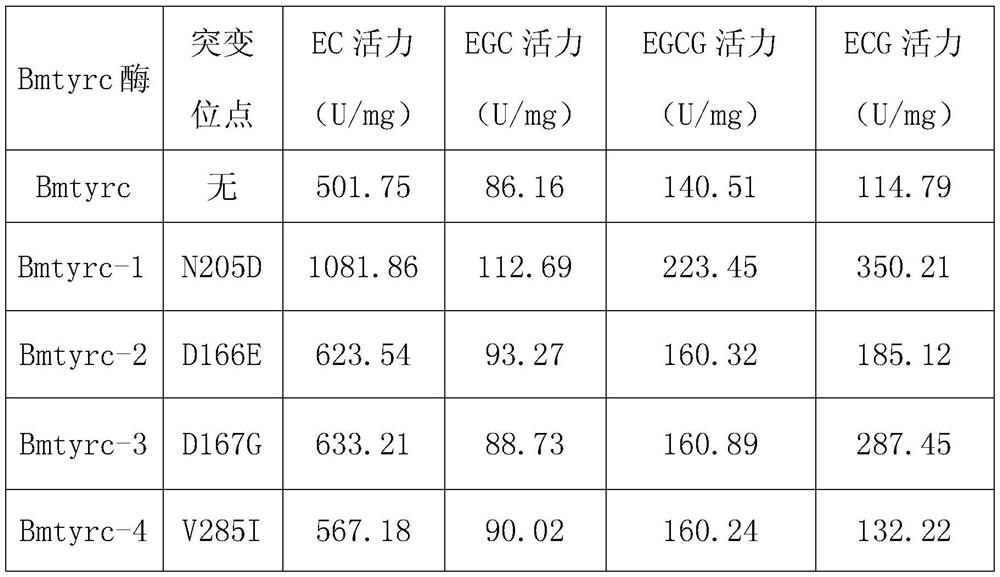
A kind of tyrosinase mutant and its application
A tyrosinase and mutant technology, which is applied in the field of tyrosinase mutants and the synthesis of theaflavins using them, and achieves the effects of high catalytic efficiency, high catalytic efficiency and conversion yield, and high specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Construction of Bacillus megaterium-derived tyrosinase Bmtyrc prokaryotic expression strain
[0037] Download the amino acid sequence of tyrosinase derived from Bacillus megaterium in GenBank (SEQ ID NO.1 in this paper, corresponding to GenBank accession number: ACC86108.1), and submit the amino acid sequence to Beijing Qingke Biotechnology Co., Ltd. for the synthesis of the whole gene sequence (using large intestine Bacillus preferred codons). The C-terminal of the synthetic gene carries a His tag and is constructed into the prokaryotic expression vector pET30a(+). Pass the constructed plasmid pET30a(+)-Bmtyrc through CaCl 2 Transform into Escherichia coli expression strain BL21(DE3) by heat shock transformation, spread on LB solid medium plate containing 50 μg / ml Kanamycin, cultivate overnight at 37°C, and the colonies grown on the plate are prokaryotic expression of tyrosinase Recombinant strain E. coli BL21(DE3) / pET30a(+)-Bmtyrc.
[0038] Use a sterili...
Embodiment 2
[0039] Example 2: Purification and Immobilization of Tyrosinase (Bmtyrc)
[0040] Using the His tag carried in the Bmtyrc recombinant protein, using activated IDA resin (purchased from Anoron (Beijing) Biotechnology Co., Ltd., specific model: His.Bind Resin, Ni-charged), the specific methods and steps used As follows: 4°C, 10000r / min, centrifuge the fermentation broth for 10min, discard the supernatant, collect the cells, wash the cells twice with phosphate buffer (pH8.0, 0.1mol / L), and concentrate the cells after centrifugation 5-fold resuspended in 20 mL of phosphate buffer (pH 8.0, 0.1 mol / L). The bacterial solution after the above treatment was placed in ice water for sonication until clarification, and the sonication conditions were: working for 2s, interval of 5s, and ultrasonic power of 500W. The fragmented lysate was centrifuged in a low-temperature high-speed centrifuge (12000 rpm, 4° C., 20 min), and the supernatant was collected to obtain crude protein. The crude ...
Embodiment 3
[0044] Example 3: Construction of Bmtyrc prokaryotic expression strain E. coli BL21(DE3) / pET30a(+)-Bmtyrc error-prone mutation library
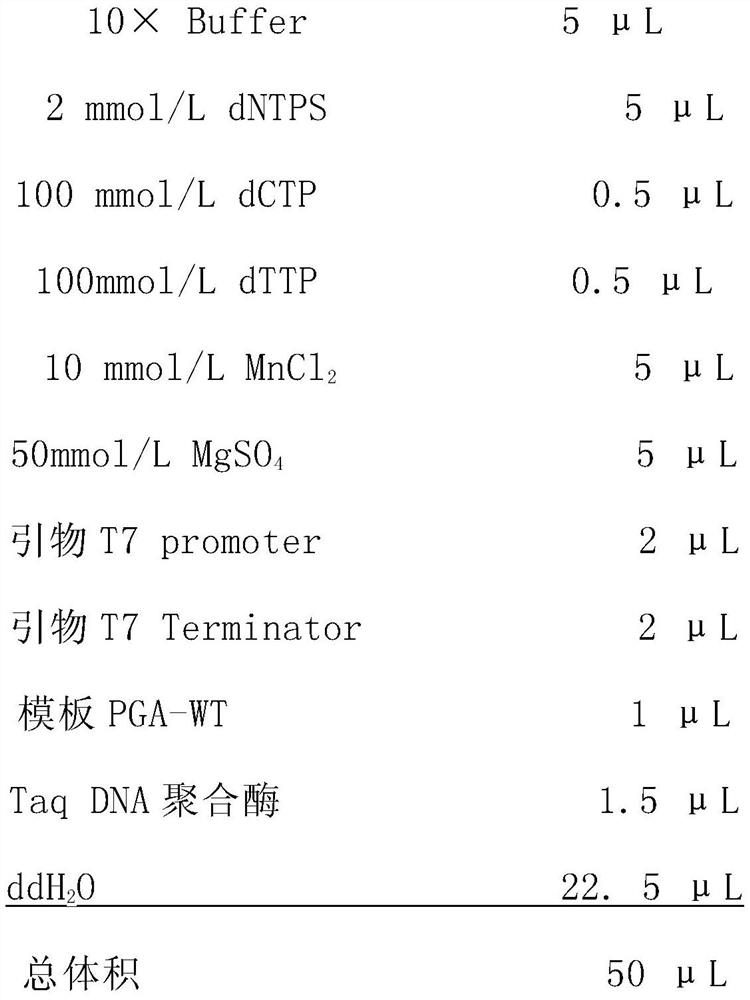
[0045] Using pET30a(+)-Bmtyrc recombinant plasmid as PCR template, conventional T7F / R as universal primer (primer sequence: T7F: 5'-TAATACGACTCACTATAGGG-3', T7R: GCTAGTTATTGCTCAGCGG see SEQ ID NO. 12 and 13) for Bmtyrc gene Perform error-prone PCR amplification and adjust Mg in the PCR amplification reaction system 2+ , Mn 2+ , dCTP and dTTP oligonucleotide concentrations, so that the base mismatch rate of the mutant library is only 2 / 1000, that is, to ensure that only 1 to 2 amino acids are mutated in a mutant.
[0046] Error-prone PCR reaction system:
[0047]
[0048] Error-prone PCR reaction conditions: pre-denaturation at 95°C for 5 min; then denaturation at 94°C for 30 s, annealing at 56°C for 1 min, extension at 72°C for 1.5 min, a total of 25 cycles; and finally extension at 72°C for 10 min.
[0049] 2 μL of the above error-pron...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com