Ahylysantinfarctase 36KD single-stranded haemocoagulase and its preparing method
A technology of hemocoagulase and Agkistrodon, which is applied in the field of separation and purification to obtain hemocoagulase and its preparation, to achieve the effects of stable preparation process, shortened coagulation time and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1, 20060418 batch of Agkistrodon acutus venom 3.6KD hemagglutinin production example
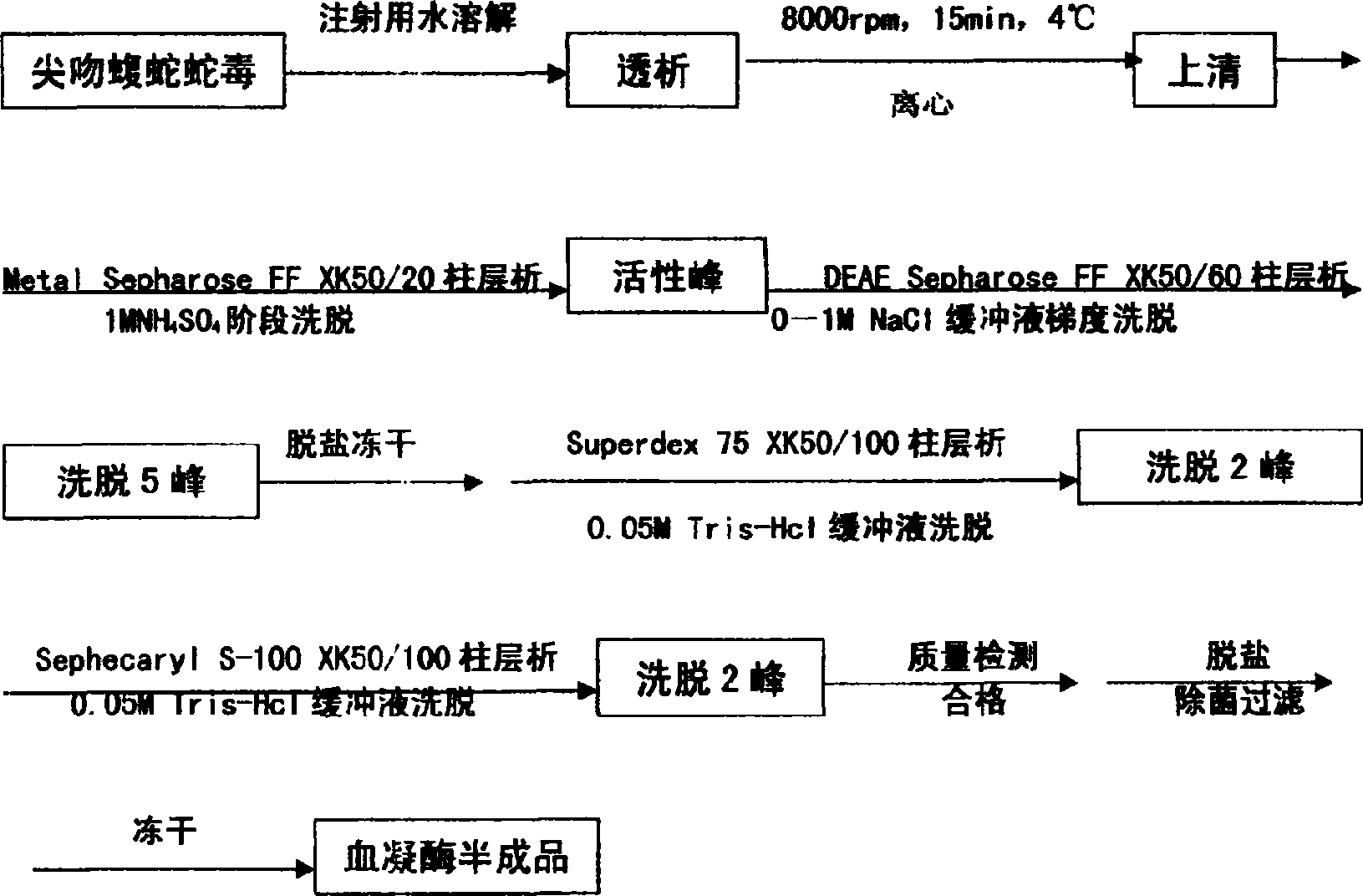
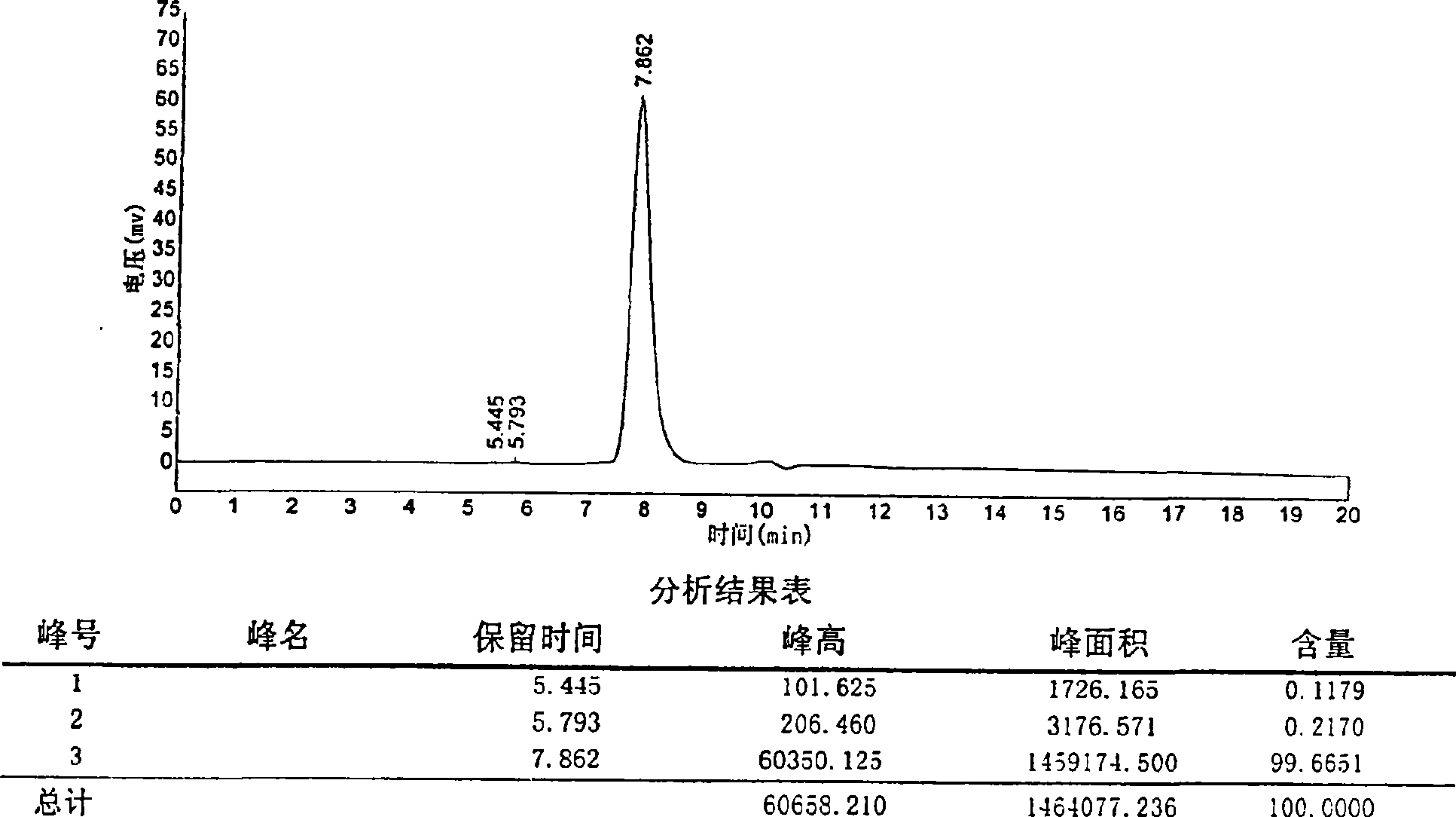
[0032] Weigh 10g of the lyophilized product of Agkistrodon acutus venom with 50ml of sterile water for injection, dialyzed overnight in deionized water at 4℃, centrifuge at 8000rpm, 15min, 4℃, and adjust the supernatant to 3.5 with 1M Tris-HCl (PH8.0). After ms / cm, load the affinity chromatography column Metal Sepharose FF, 1M NH, which is pre-equilibrated with 0.05M Tris-HCl (PH8.0) 4 Cl carries out phase elution and collects the breakthrough peak; the balanced DEAE-Sepharose column on the breakthrough peak, 0.05M Tris-HCl (PH8.0) zero wash 1 times the column bed volume, use 0.05M Tris-HCl+1.0 M NaCl (PH8.0) was used for gradient elution. Five peaks (ie crude hemagglutinin fraction) were collected under UV detection at 280nm, and the active peak was desalted and lyophilized. The lyophilized product was dissolved in 10-20ml 0.05M Tris- HCl(PH7.5)+0.1M KCl, pre-equilibrated with 0....
Embodiment 2
[0033] Example Two, 20060428 batch of Agkistrodon acutus venom 3.6KD hemagglutinin production example
[0034] Weigh 10g of the lyophilized product of Agkistrodon acutus venom with 50ml of sterile water for injection, dialyzed overnight in deionized water at 4℃, centrifuge at 8000rpm, 15min, 4℃, and adjust the supernatant to 3.5 with 1M Tris-HCl (PH8.0). After ms / cm, load the affinity chromatography column Metal Sepharose FF column, 1M NH, which is pre-equilibrated with 0.05M Tris-HCl (PH8.0) 4 Cl carries out phase elution, and collects the breakthrough peak; the DEAE-Sepharose column is well balanced on the breakthrough peak, and after 0.05M Tris-HCl (PH8.0) zero wash 1 column bed volume, use 0.05M Tris-HCl+1.0 M NaCl (PH8.0) was used for gradient elution, 5 peaks (ie crude hemagglutinin fraction) were collected under UV 280nm detection, and the active peak was desalted and lyophilized. The lyophilized product is dissolved in 10-20ml 0.05M Tris-HCl(PH7.5)+0.1M KCl, and the Superd...
Embodiment 3
[0035] Example three, 20060523 batch of Agkistrodon acutus venom 3.6KD hemagglutinin production example
[0036]Weigh 10g head of the lyophilized Agkistrodon acutus venom with 50ml sterile water for injection, dialyze overnight in deionized water at 4°C, centrifuge at 8000rpm, 15min, 4°C, and adjust the supernatant with 1M Tris-HCl (PH8.0) to adjust the conductivity to After 3.3ms / cm, load the Metal Sepharose F.FXK50 / 30 (product of GE Healthcare), 1M NH, which has been pre-balanced with 0.05MTris-HCl (PH8.0) 4 Cl carries out phase elution, and collects the breakthrough peak; the DEAE-Sepharose column is well-balanced on the breakthrough peak, and after 0.05M Tris-HCl (Ph8.0) zero wash 1 column bed volume, use 0.05M Tris-HCl+1.0 M NaCl (PH8.0) is used for gradient elution. 5 elution peaks (ie crude hemagglutinin fraction) are collected under UV 280nm detection, and the activity peak is desalted and lyophilized. The lyophilized product is dissolved in 10-20ml 0.05M Tris-HCl (PH7.5)+...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com