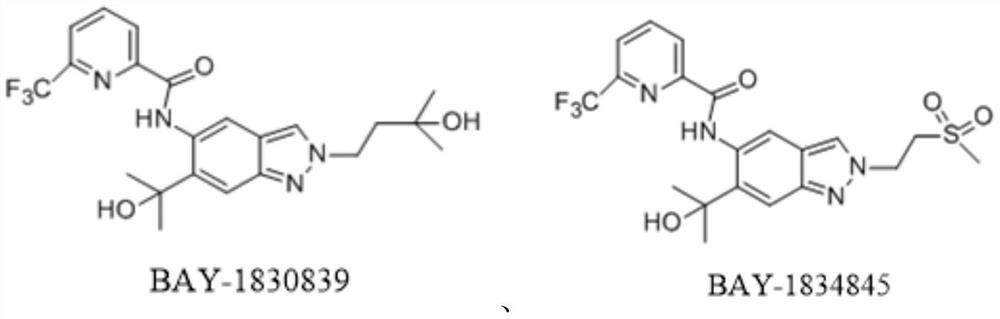
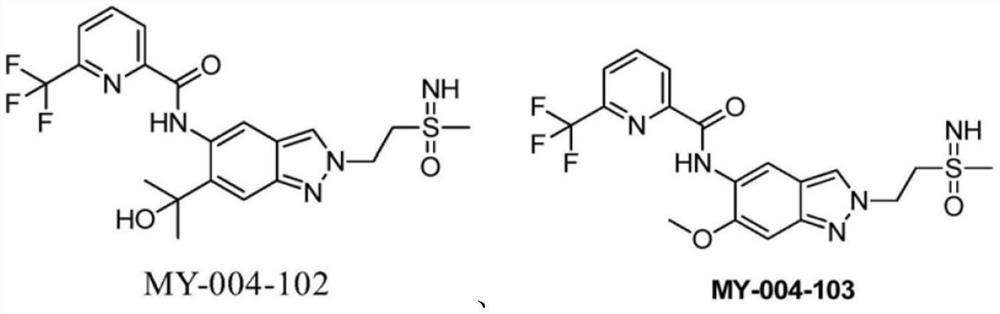
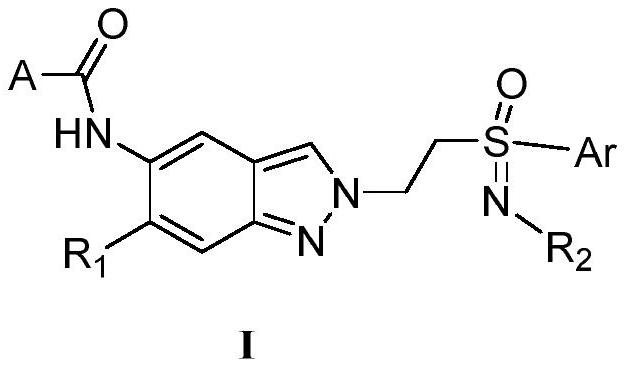
A kind of indazole irak4 kinase inhibitor substituted by sulfenimide, preparation method and application
A technology of sulfinimides and indazoles, applied in the field of biomedicine, can solve the problems of unsatisfactory efficacy, safety, pharmacokinetics, low pharmacokinetics and bioavailability, and animal safety risks To achieve good pharmacokinetic properties, reduce the risk of hERG inhibition, and good IRAK4 inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the synthesis of I-1
[0077] synthetic route:
[0078]
[0079] Steps:
[0080] step 1:
[0081] Compound IA-1 (1.63g, 0.01mol), IB-1 (1.91g, 0.01mol), add dichloromethane (DCM, 30mL), then add N,N-diisopropylethylamine (DIPEA, 1.94 g, 0.015mol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDCI) (2.3g, 0.015mol), and the reaction system was stirred at 30°C for 12h. The reaction mixture was extracted with water (20 mL), the organic layer was concentrated to dryness under reduced pressure, and recrystallized by adding absolute ethanol (10 mL) to obtain IC-1 as a light yellow solid (2.67 g, yield 79.5%). 1 HNMR (400MHz, DMSO-d 6 ):δ=13.10(br,1H),11.55(br,1H),8.35(d,J=8.0Hz,1H),8.14(m,1H),7.98(d,J=8.0Hz,1H),7.72 (s,1H),7.47(s,1H),7.04(s,1H),3.92(s,3H). LCMS: MS Calcd.: 336.3, MS Found: 337.2 [M+1].
[0082] Step 2:
[0083] Compound IC-1 (400mg, 1.2mmol), ID-1 (327mg, 1.32mmol), K 2 CO 3 (332mg, 2.4mmol), KI (17mg, 0.1mmol) were added in DMF (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com