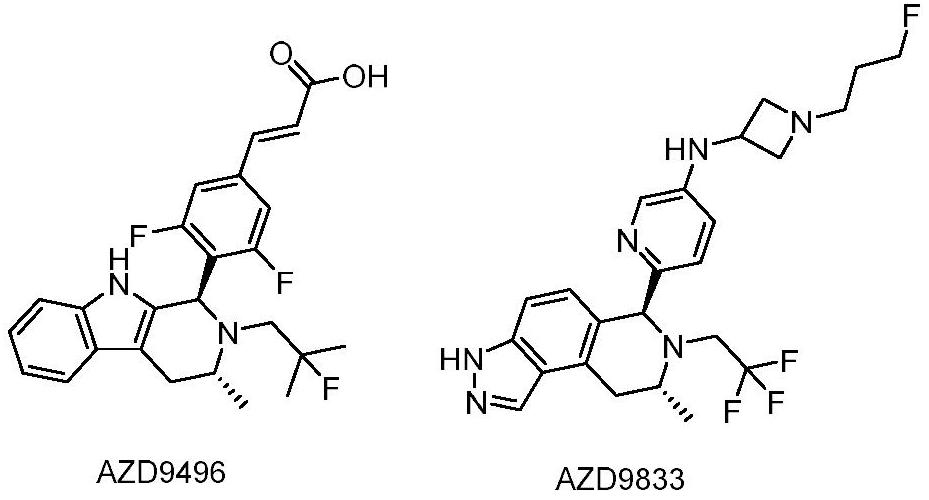
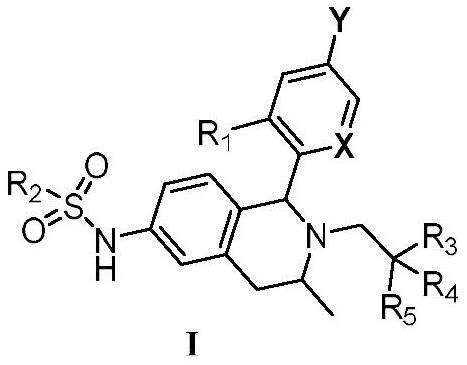
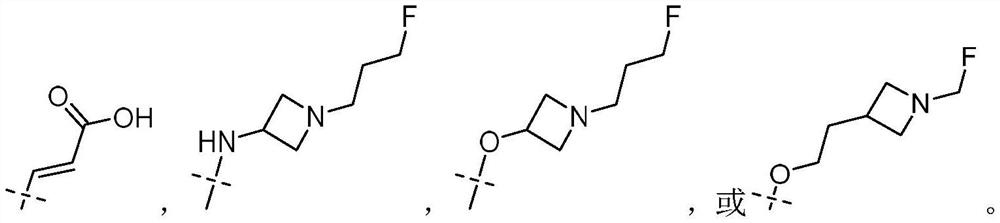
Tetrahydroisoquinoline compounds as selective estrogen receptor down-regulator, synthesis method and application
An estrogen receptor and synthesis method technology, applied in the field of its preparation and tetrahydroisoquinoline derivatives, can solve the problems of poor drug characteristics, limited effective degradation of ER, etc., and achieves low effective dose, excellent SERD activity, low Effects of security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~16
[0065] The synthesis of embodiment 1~16:I-1
[0066] synthetic route:
[0067]
[0068] step 1:
[0069] Compound IA-1 (2.28g, 0.01mol), IB-1 (2.24g, 0.01mol) was added to 1,4-dioxane (40mL), and then N,N-diisopropylethylamine (DIPEA , 3.87g, 0.03mol), the reaction system was stirred at 80°C for 12h. The reaction solution was concentrated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether 1 / 1) to obtain the target product IC-1 (1.36 g, yield 45%). LCMS: MS Calcd.: 302.4, MS Found: 303.2 [M+1].
[0070] Step 2:
[0071] Compound IC-1 (500mg, 1.65mmol), ID-1 (411mg, 1.82mmol) and acetic acid (500mg, 8.3mmol) were added to toluene (10mL), under the protection of nitrogen, the reaction system was heated to 80°C and stirred for 12h. The reaction solution was concentrated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: ethyl a...
Embodiment 17-40
[0081] synthetic route:
[0082]
[0083] step 1:
[0084] Compound IA-1 (2.28g, 0.01mol), IB-1 (2.24g, 0.01mol) was added to 1,4-dioxane (40mL), and then N,N-diisopropylethylamine (DIPEA , 3.87g, 0.03mol), the reaction system was stirred at 80°C for 12h. The reaction solution was concentrated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether 1 / 1) to obtain the target product IC-1 (1.36 g, yield 45%). LCMS: MS Calcd.: 302.4, MS Found: 303.2 [M+1].
[0085] Step 2:
[0086] Compound IC-1 (500mg, 1.65mmol), IF-1 (383mg, 1.82mmol) and acetic acid (500mg, 8.3mmol) were added to toluene (10mL), under the protection of nitrogen, the reaction system was heated to 80°C and stirred for 12h. The reaction solution was concentrated to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether 1 / 3) to obtain the targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com