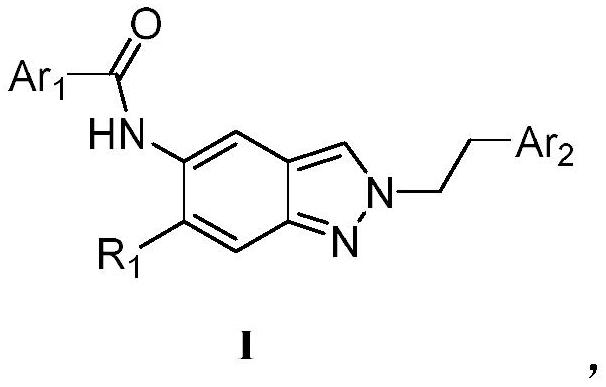
Substituted indazole compound, preparation method, application and composition containing substituted indazole compound
A technology of compounds and indazoles, applied in drug combination, metabolic diseases, organic chemistry, etc., can solve animal safety risks, effectiveness, unsatisfactory safety pharmacokinetics, pharmacokinetics and biological Problems such as low utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
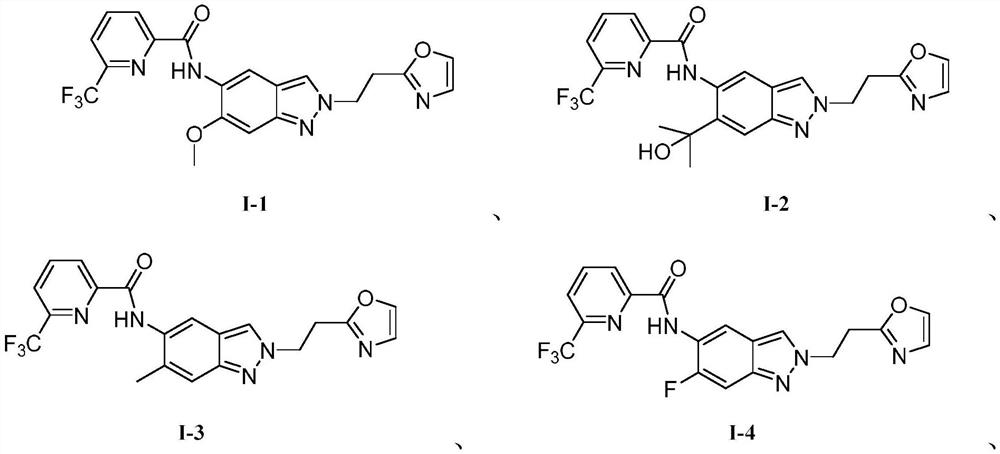
[0034] Embodiment 1: the synthesis of I-1 (replacing compound with the general formula label of structural formula, the same below)
[0035] A kind of substituted indazole compound, has the structure shown in general formula (I-1), and synthetic route is specifically as follows:
[0036]
[0037] The specific steps are:
[0038] S1: Add compound Ia-1 (1.63g, 0.01mol) and compound Ib-1 (1.91g, 0.01mol) into dichloromethane (30mL), then add N,N-diisopropylethylamine (DIPEA, 1.94g, 0.015mol), benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU, 5.7g, 0.015mol), the reaction system was stirred at 30°C for 12h . The reaction mixture was extracted with water (20 mL), the organic layer was concentrated to dryness under reduced pressure, and recrystallized by adding absolute ethanol (10 mL) to obtain a pale yellow solid Ic-1 (2.85 g, yield 85%). The hydrogen spectrum of Ic-1 is 1 HNMR (400MHz, DMSO-d 6 ): δ=13.10(br,1H), 11.55(br,1H), 8.35(d,J=8.0Hz,1H), 8.14(...
Embodiment 2
[0040] The synthesis of embodiment 2:1-2
[0041] A substituted indazole compound has a structure shown in general formula (I-2), and the synthetic route is specifically as follows:
[0042]
[0043] Except reaction raw material Ia-2, all the other operation steps are all identical with embodiment 1, and the yield of product I-2 is 15%, and hydrogen spectrum is 1 HNMR (400MHz, DMSO-d 6 ):δ=11.56(br,1H),8.38(d,J=8.0Hz,1H),8.15(m,2H),8.01(s,1H),7.95(d,J=8.0Hz,1H),7.85 (s, 1H), 7.75 (d, J = 8.4Hz, 1H), 7.23 (d, J = 8.4Hz, 1H), 5.56 (br, 1H), 4.12 (t, J = 4.8Hz, 2H), 3.05 (t, J=4.8Hz, 2H), 1.38(s, 6H). LCMS: MS Calcd.: 459.4, MS Found: 460.3 [M+1].
Embodiment 3
[0044] The synthesis of embodiment 3:1-3
[0045] A kind of substituted indazole compound, has the structure shown in general formula (I-3), and synthetic route is specifically as follows:
[0046]
[0047] Except reaction raw material Ia-3, all the other operation steps are all the same as embodiment 1, and the yield of product I-3 is yield 13%, and hydrogen spectrum is 1 HNMR (400MHz, DMSO-d 6 ):δ=11.53(br,1H), 8.35(d,J=8.0Hz,1H), 8.13(m,2H), 8.01(s,1H),7.96(d,J=8.0Hz,1H),7.88 (s, 1H), 7.73(d, J=8.4Hz, 1H), 7.23(d, J=8.4Hz, 1H), 4.15(t, J=4.8Hz, 2H), 3.09(t, J=4.8Hz ,2H), 2.10(s,3H). LCMS: MS Calcd.: 415.3, MS Found: 416.3 [M+1].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com