Methods and systems for in vivo full-field interference microscopy imaging
An imaging system and full-field technology, applied in the fields of eye testing equipment, medical science, diagnosis, etc., can solve the problems of predetermined phase offset, FFOCT signal degradation, and inability to construct 3D images, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
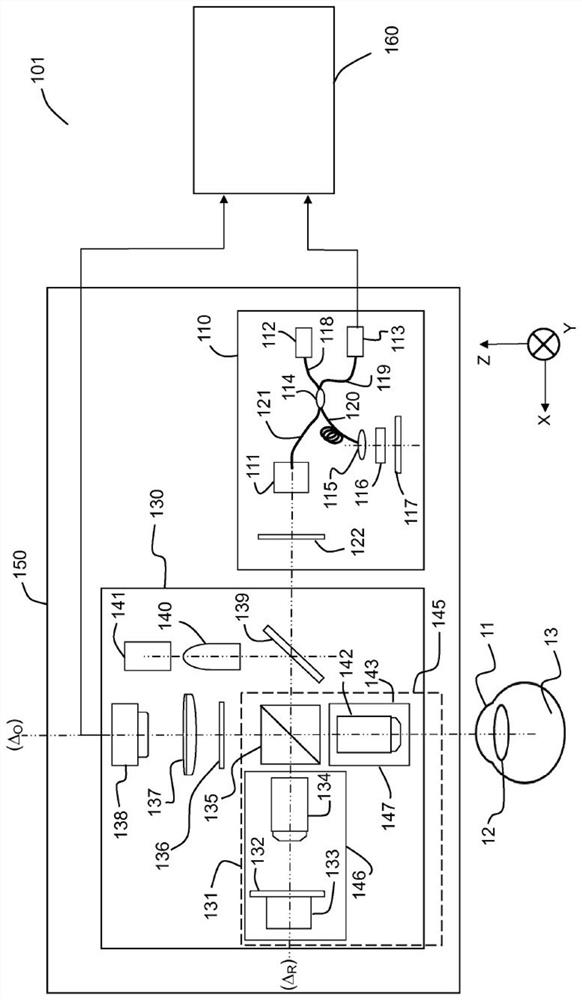
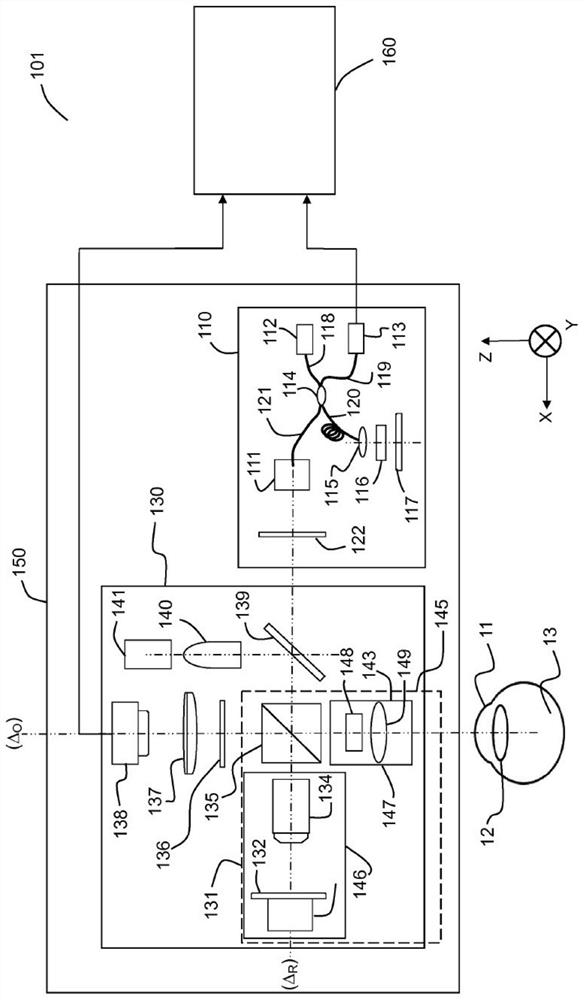
[0067] system
[0068] Figure 1A and Figure 1B Two embodiments 101 , 102 of systems for in vivo full-field interferometric microscopy imaging according to the present specification are shown respectively. The system 101 is suitable for implementing a method for 3D imaging of a moving sample in vivo, especially (but not limited to) the anterior portion 11 (cornea) of the eye in vivo. The system 102 is suitable for implementing a method for 3D imaging of a moving sample in vivo, especially (but not limited to) the posterior portion 13 (retina) of the eye in vivo.
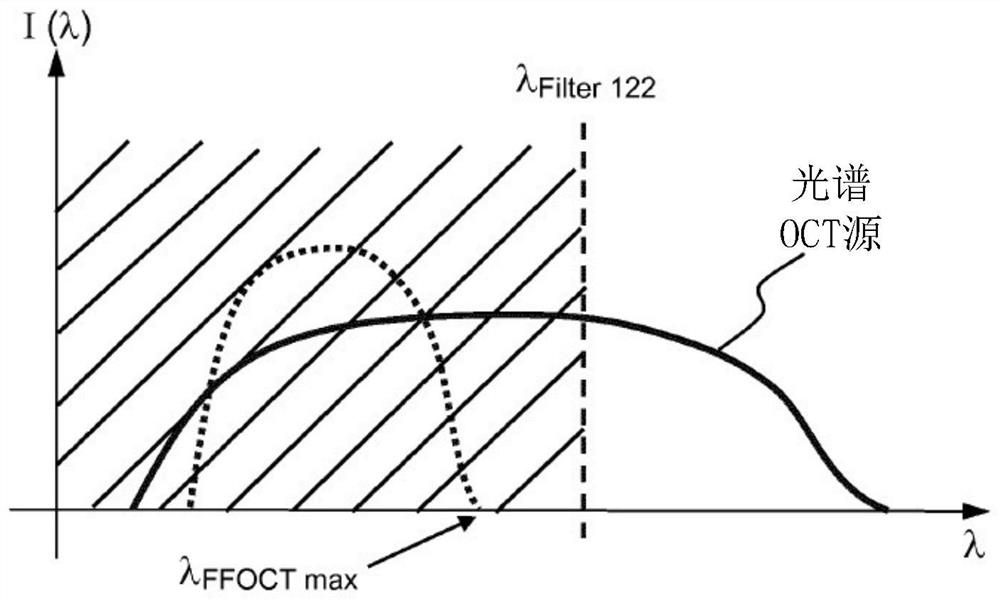
[0069] Figure 1A The illustrated system 101 includes two imaging systems, a full-field OCT (“FFOCT”) imaging system 130 and an optical coherence tomography (“OCT”) imaging system 110 , and at least one processing unit 160 . An FFOCT imaging system is capable of acquiring a "frontal" image of a moving in vivo sample 11, i.e. an image of a deep section of the sample, and an optical coherence tomography ("OCT") im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com