A fluorescent hydrogel and its application in the detection of carbaryl
A hydrogel and carbaryl technology, applied in the field of biosensors, can solve problems such as interference, achieve high stability and selectivity, and avoid background fluorescence interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
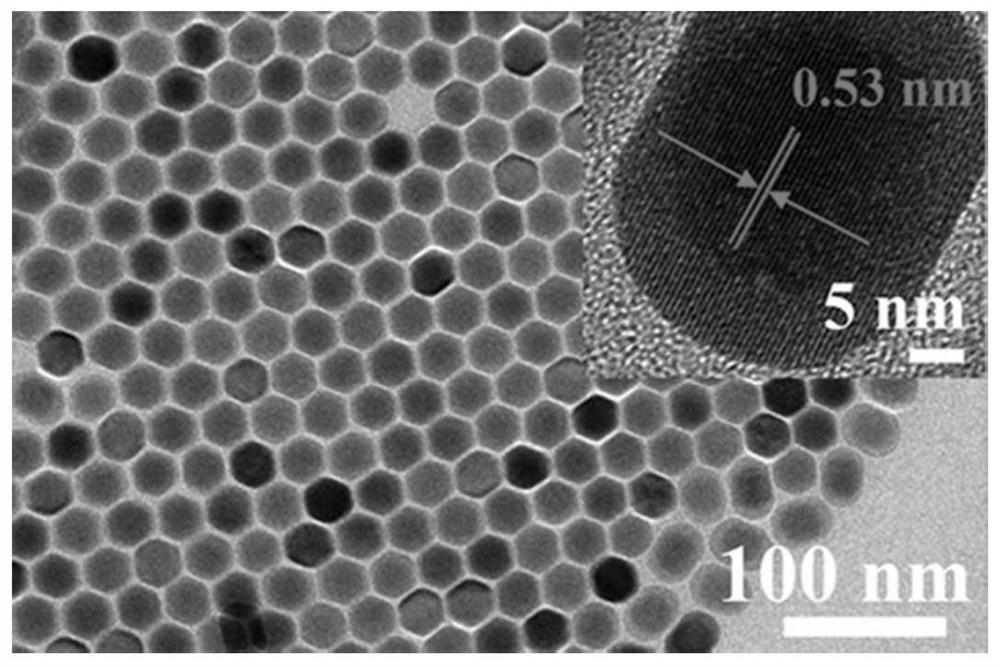
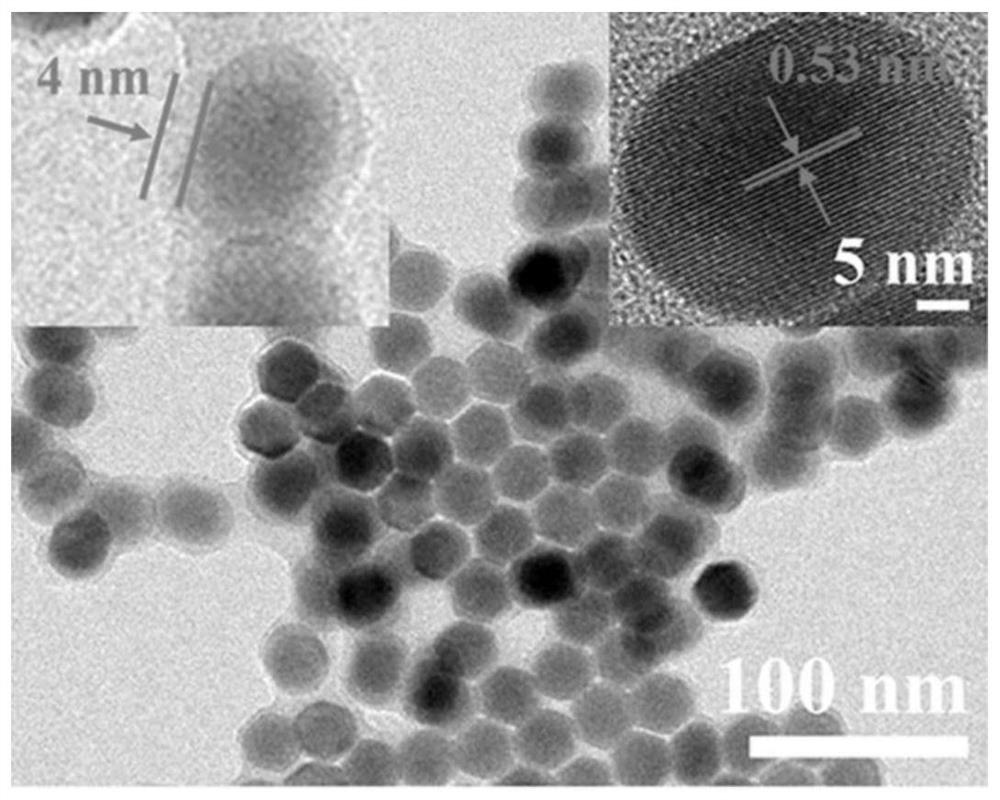
[0019] Weigh 0.37980g erbium trichloride hexahydrate and 0.00192g thulium trichloride hexahydrate and join in the three-neck round bottom flask of 50mL, then add 6mL oleic acid and 15mL 1-octadecene to it, at 700r min -1 Nitrogen was introduced under stirring for 30 minutes to remove oxygen, and then heated to 160°C and continued to stir for 25 minutes until a pale pink precursor solution was formed; after the precursor solution was cooled, 10 mL of methanol solution containing 0.1 g of sodium hydroxide and Add dropwise to the above solution, heat to 75°C to remove methanol; heat the system to 300°C for 90 minutes, and then -1 Centrifuge at a rotating speed, wash with a mixture of cyclohexane and ethanol (the volume ratio of cyclohexane and ethanol is 3:1) for 4 times, and then dissolve in cyclohexane to obtain solution A. Weigh 0.15168g of yttrium trichloride hexahydrate and add it into 6mL of oleic acid and 15mL of 1-octadecene, stir for 30 minutes under nitrogen protection,...
Embodiment 2
[0020] Embodiment 2: a kind of based on NaErF 4 :0.5%Tm 3+ @NaYF 4 The preparation method of / PDA fluorescent hydrogel and its application in carbaryl detection comprise the following steps:
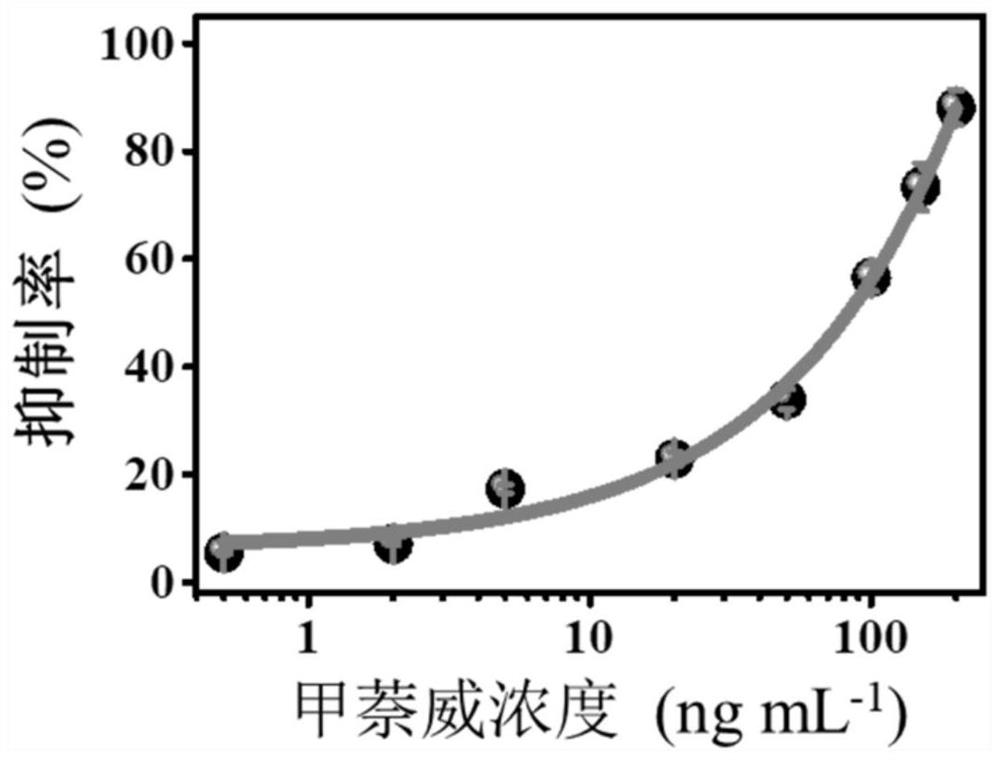
[0021] 50μL, 20ng mL -1 Carbaryl standard solution with 50μL, 2.5U L -1 The AChE was reacted at 37 °C for 30 min. It is added to the NaErF that contains embodiment 1 preparation 4 :0.5%Tm 3+ @NaYF 4 / PDA fluorescent hydrogel cuvette, react at 37°C for 30 minutes. Fluorescent images of different carbaryl concentrations were obtained by irradiating the cuvette with a small 980nm laser, and were analyzed and processed by ImageJ software. At the concentration of carbaryl in this example, the corresponding inhibition rate of the sample was 22.89%.
Embodiment 3
[0022] Embodiment 3: a kind of based on NaErF 4 :0.5%Tm 3+ @NaYF 4 The preparation method of / PDA fluorescent hydrogel and its application in carbaryl detection comprise the following steps:
[0023] 50μL, 100ng mL -1 Carbaryl standard solution with 50μL, 2.5U L -1 The AChE was reacted at 37 °C for 30 min. It is added to the NaErF that contains embodiment 1 preparation 4 :0.5%Tm 3+ @NaYF 4 / PDA fluorescent hydrogel cuvette, react at 37°C for 30 minutes. Fluorescent images of different carbaryl concentrations were obtained by irradiating the cuvette with a small 980nm laser, and were analyzed and processed by ImageJ software. Under the concentration of carbaryl in this example, the corresponding inhibition rate of the sample is 56.54%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com