Pharmaceutical composition for preventing and treating intestinal colibacillosis of yak calves and preparation method of pharmaceutical composition
A technology for colibacillosis and a composition, which is applied in the field of veterinary medicine, can solve problems such as drug resistance of pathogenic Escherichia coli, public health threats, and drug residues in feces, and achieves the advantages of protecting biological activity, reducing the number of Escherichia coli, and being convenient to take. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation method of embodiment 1 pharmaceutical composition, the steps are as follows:
[0020] Materials: Artemisinin (CAS63968-64-9, Lot#C10420277, ≧98%), purchased from Shanghai Macklin Biochemical Technology Co., Ltd.; Sodium Selenite Tablets (1.0mg / tablet, National Drug Approval H37023197, Lot No. 20190845) , purchased from Shandong Xili Pharmaceutical Co., Ltd.; ferrous sulfate (analytical pure AR, CAS7782-63-0, batch number 20190722), purchased from Sinopharm Chemical Reagent Co., Ltd.; shell powder (food grade, 600 mesh, batch number 20190516) , purchased from Hebei Huihao Environmental Protection Technology Co., Ltd.; mannan oligosaccharide (food grade, purity 99%, product number 15516382283), purchased from Zhengzhou Best Food Additive Co., Ltd.; antibiotic norfloxacin (NOR, CAS70458-96- 7. Lot#N814880, ≧98%), purchased from Shanghai Macklin Biochemical Technology Co., Ltd.; Ampicillin Sodium (AMP, Lot. No. 826H031, ≧85%), purchased from Beijing Suo Laib...
Embodiment 2 4
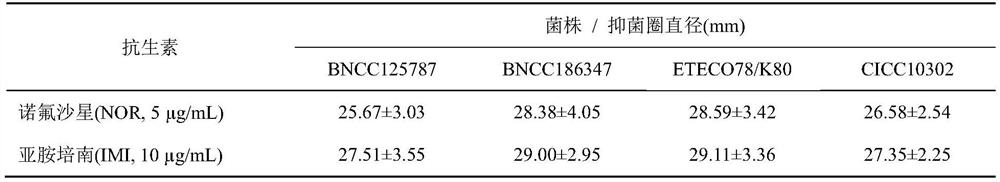
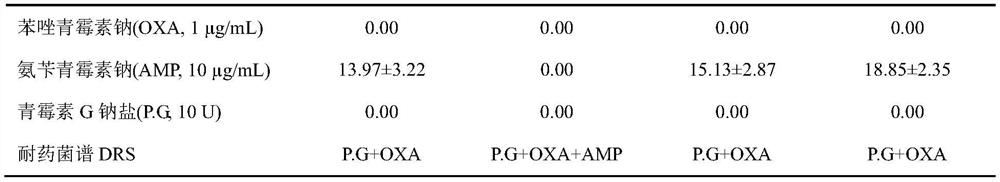
[0047] The drug susceptibility of embodiment 2 four strains of escherichia coli and the drug-resistant bacterial spectrum determination (K-B method)
[0048] Follow the steps recommended by the National Committee for Clinical Laboratory Standards (NCCLS). Test strain: Escherichia coli (E.coli, ETEC-O 78 K 80 , CICC10421), Escherichia coli (E.coli, CICC10302), purchased from China Industrial Microorganism Culture Collection Management Center; Escherichia coli (E.coli, BNCC125787), Escherichia coli (E.coli, BNCC186347) , purchased from Beijing Beina Chuanglian Biotechnology Research Institute.
[0049] (1) A single colony of Escherichia coli freshly cultivated was prepared with 0.45% sterile physiological saline solution to prepare a suspension of the tested bacteria at a concentration equivalent to 0.5 McFarland. Spread it evenly on the surface of MH nutrient agar to prepare an MH agar plate containing bacteria to be tested.
[0050] (2) Paste the drug-sensitive paper sheet...
Embodiment 3
[0056] Example 3 Study on the combined antibacterial and sensitizing effect of artemisinin and 2 kinds of antibiotics
[0057] (1) Determination of MIC values of artemisinin and 2 kinds of antibiotics against 4 strains of pathogenic Enterobacteriaceae (96-well plate micro broth dilution method and E-test)
[0058] Follow the microdilution steps recommended by the National Committee for Clinical Laboratory Standards (NCCLS). Pipette the MH broth into the NEST 96-well plate, 100 μL per well. Add 100 μL of the drug solution to the first well of the above-mentioned NEST 96-well plate containing MH broth, blow and mix with an exhaust gun, and then dilute to the 11th hole sequentially by the doubling dilution method, and the 12th hole does not add the drug as a positive control. Add 100 μL of the prepared bacterial solution to each well of the 1st to 10th and 12th columns of the NEST 96-well plate, and no bacteria were added to the 11th well as a negative control. Add bacterial ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap