Benzimidazole compounds and use thereof for treating alzheimer's disease or huntington's disease
A compound, benzene ring technology, applied in the field of treatment of Alzheimer's disease or Huntington's disease, can solve problems such as uncontrolled movement of arms, face and upper body, decline in thinking and reasoning ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
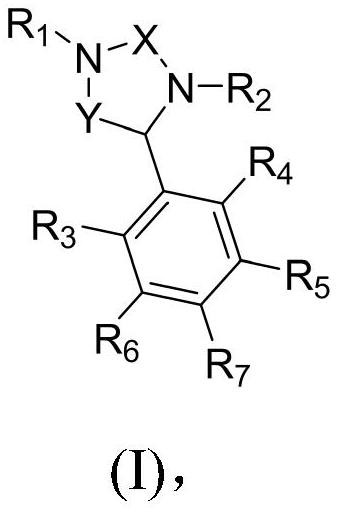
[0030] Disclosed in detail below are the benzimidazole compounds of formula (I) shown above.
[0031] The compounds of the present invention having chiral centers may exist as stereoisomers. Stereoisomers of compounds of formula (I) may include cis and trans isomers, optical isomers such as (R) and (S) enantiomers, diastereoisomers, geometric isomers, rotamers Isomers, atropisomers, conformers and tautomers of such compounds, including compounds exhibiting more than one type of isomerism; and mixtures of such isomers (e.g. racemate and mixtures of diastereomers). All such isomeric forms are contemplated. Furthermore, the compounds of formula (I) in the present invention may exhibit tautomerism.
[0032] Notably, compounds of formula (I) may have an enantiomeric excess of 90% or more (eg > 95% and > 99%).
[0033] Table 1 below shows 117 exemplary compounds of formula (I):
[0034]
[0035]
[0036]
[0037]
[0038]
[0039] Table 1
[0040] Among the 117 ...
Embodiment 1
[0046] Example 1: Preparation and Characterization of Compounds
[0047] Compounds 1-67 were prepared by Synthetic Methods 1-18 shown in Schemes 1-18 below, respectively.
[0048] Synthetic method 1
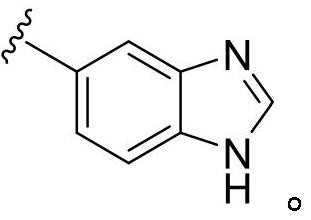
[0049] Compounds 1-4 each having a 1,3-thiazol-2-yl ring were prepared according to the synthetic procedure shown in Scheme 1 below. Coupling of (4-formylphenyl)boronic acid 118 with 2-bromothiazole derivatives 119a-d under Suzuki-coupling conditions gave 4-(1,3-thiazol-2-yl)benzaldehyde derivatives Objects 120a-d. The core structure of the imidazolidinone ring was constructed in the following three steps. TMSCN was added to a solution of benzaldehydes 120a-d and 1H-benzimidazol-5-amine 121 in acetic acid. The reaction mixture was stirred at room temperature for 2 hours and then worked up to give the aminoacetonitriles 122a-d, which were hydrogenated in acetic acid at 5-10 °C using Raney Nickel catalyst to give the diamines 123a-d. In a final step, 1,1'-carbonyldiimidazole ...
Embodiment 2
[0760] Example 2: In vitro activity screening of compounds
[0761] QC activity test
[0762] Enzyme activity assays for QC were performed at 25°C using a fluorescent substrate (ie, L-glutamine 2-naphthylamide (Gln-βNA)). See Huang et al., Biochem. J. 2008, 411, 181-190. Prepare 100 μl of reaction mixture. The reaction mixture contained 300 μM fluorescent substrate, about 0.2 units of auxiliary enzyme human pyroglutaminylamino peptidase I (PAPI) (one unit was defined as the hydrolysis of 1 μmol of pGlu-βNA per minute under the same experimental conditions) amount of human PAPI), and an appropriately diluted aliquot of recombinant QC in 50 mM Tris-HCl at pH 8.0. Excitation and emission wavelengths were set at 320 nm and 410 nm, respectively. The reaction was initiated by adding QC. The enzymatic activity of QC was determined by the amount of βNA released and calculated using a standard curve of βNA under the same experimental conditions. Measurements were performed using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com