Idiopathic thrombocytopenic purpura patient report clinical outcome evaluation system
An evaluation system and platelet technology, applied in medical reports, electronic clinical trials, instruments, etc., can solve the problems of wasting diagnostic resources and time, overlapping symptom classification, affecting disease recognition and treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 2 example
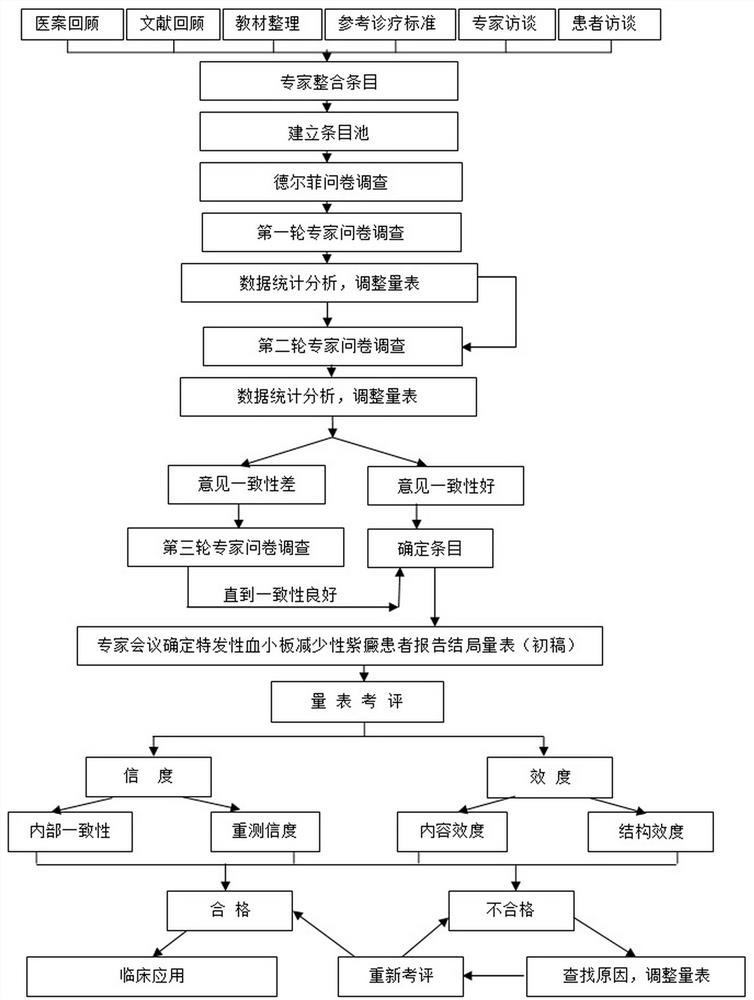
[0262] Second embodiment: preliminary evaluation of PRO scale.
[0263] 1. Research purpose
[0264] Preliminary evaluation of the reliability and validity of the patient-reported outcome scale for idiopathic thrombocytopenic purpura.
[0265] 2. Research content
[0266] The evaluation of the scale generally includes reliability analysis and validity analysis. Based on the data from the above-mentioned on-site investigations, we assessed the reliability and validity of the initially formed scale. The pre-investigation was carried out in 4 sub-centers to evaluate the reliability and validity of the scale. Through the method of scale research, gradually develop and try to establish a patient-reported outcome scale for idiopathic thrombocytopenic purpura.
[0267] 3. Overall design.
[0268] Test basis and principles;
[0269] ●Project mission statement supported by the Ministry of Science and Technology
[0270] ●Medical Ethics
[0271] ●Medical statistics experts invol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com