Recombinant protein for eliminating boar taint and vaccine composition comprising same
A vaccine composition and recombinant protein technology are applied in the field of vaccine compositions for eliminating male odor and can solve the problem of high cost and expenditure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: Expression and refining of BiP-CTB-GnRH-pFc2-HDEL fusion protein
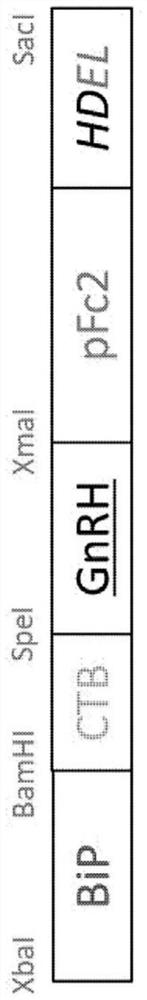
[0097] To induce the immunogenicity of GnRH, as figure 1 As shown, a vector for expressing recombinant GnRH in which CTB, pFc2, BiP and HDEL were fused to GnRH was produced. Six GnRHs consisting of 10 amino acids are connected consecutively by alanine-glycine-alanine (AGA) linkers, and the amino acid sequences and base sequences of the six connected GnRHs are shown in SEQ ID NO: 2 and SEQ ID NO: 3, respectively. . In addition, the amino acid sequence and base sequence of CTB are shown in SEQ ID NO: 4 and SEQ ID NO: 5, the amino acid sequence and base sequence of pFc2 are shown in SEQ ID NO: 6 and SEQ ID NO: 7, respectively, and the amino acid sequence and base sequence of BiP They are shown in SEQ ID NO: 8 and SEQ ID NO: 9, respectively, and the amino acid sequence and base sequence of HDEL are shown in SEQ ID NO: 10 and SEQ ID NO: 11, respectively. Meanwhile, the entire amino acid seq...
Embodiment 2
[0099] Embodiment 2: confirmation of recombinant GnRH protein concentration
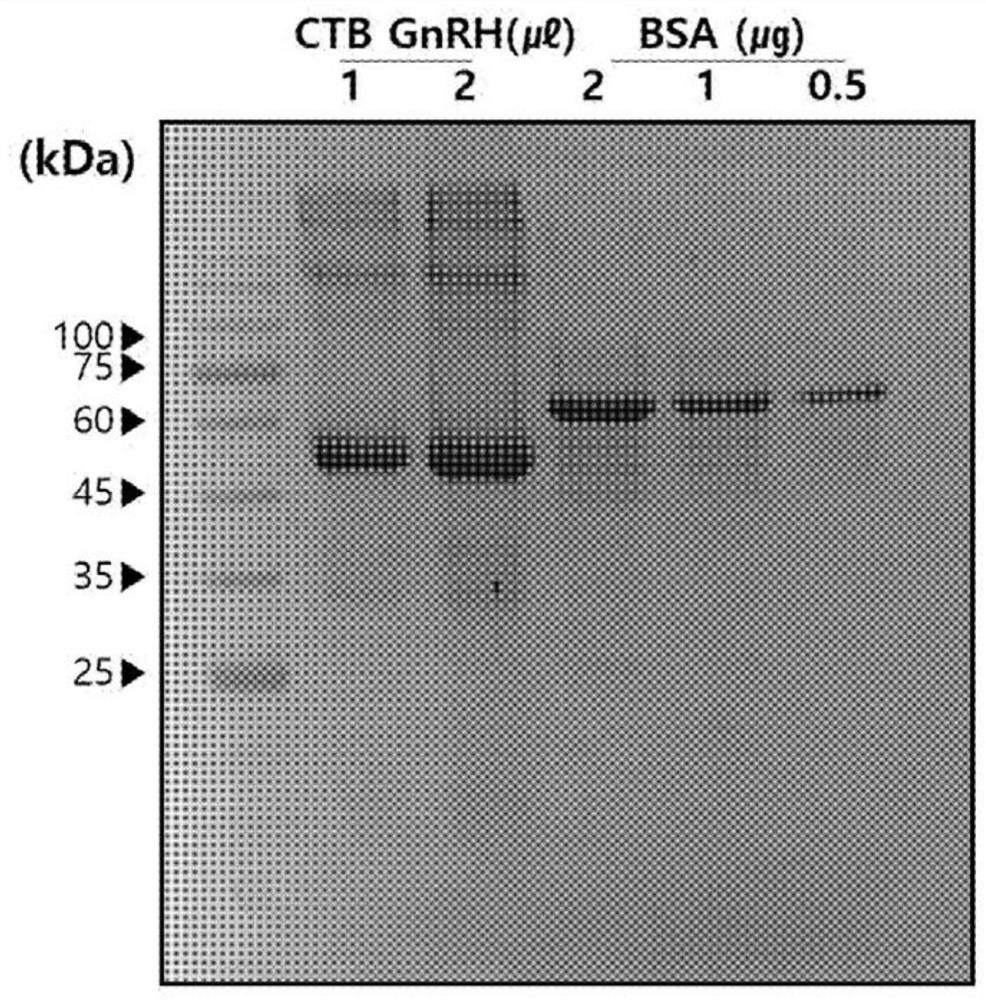
[0100] In order to confirm and measure the concentration of the recombinant GnRH protein isolated and refined in Example 1, bovine serum albumin (BSA, bovine serum albumin) of known concentration was used as a standard (standard), and SDS-PAGE and Brasil were carried out. Deford quantitative analysis (bradford assay). After loading 1 and 2 μl of the isolated and refined recombinant GnRH protein, 0.5, 1 and 2 μg of BSA were contained therein for quantitative processing. Gel finally confirmed the protein band by Coomassie blue staining (Coomassie bluestaining). Bradford Quantitative Analysis used BSA as a standard to draw a standard curve (standard curve) and formulate it, and then substituted the UV value of GnRH to calculate the concentration.
Embodiment 3
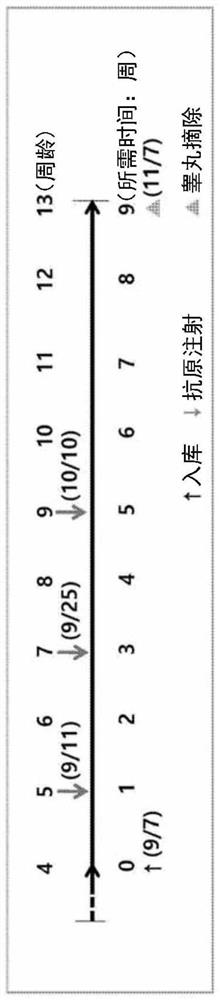
[0102] Embodiment 3: the injection effect confirmation of recombinant GnRH protein
[0103] 3.1. Experimental animals, injection conditions and schedule
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com