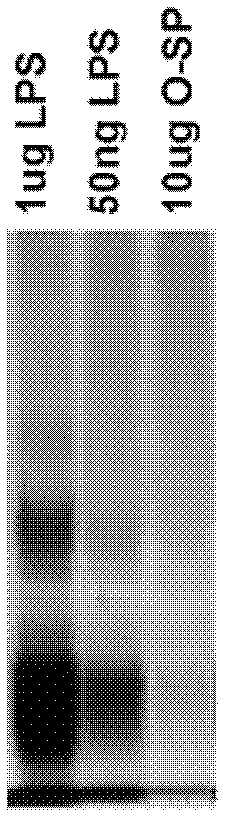
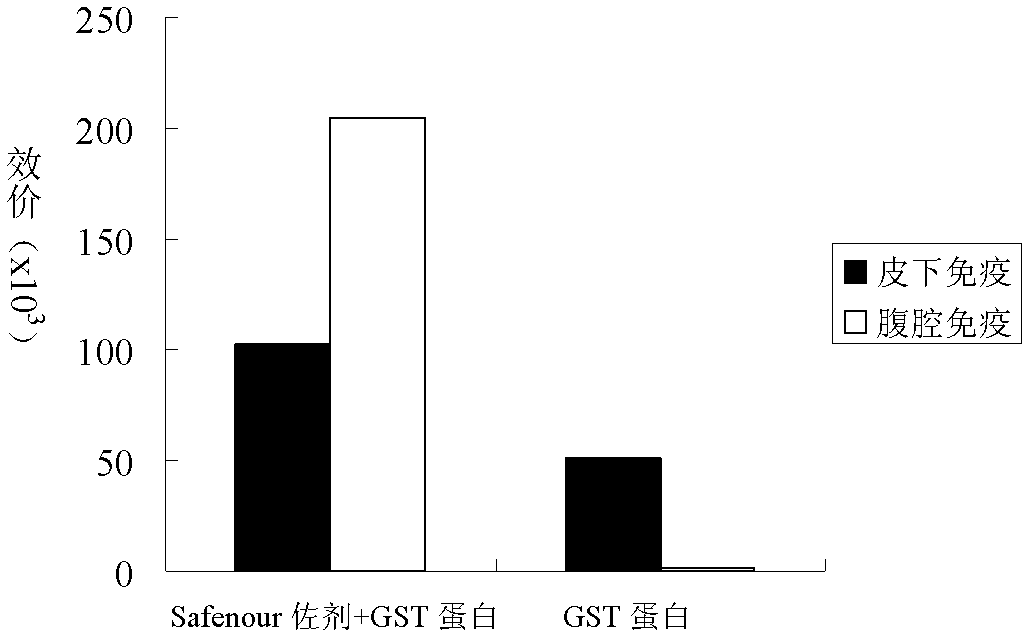Patents
Literature
70 results about "Cholera Toxin B Subunit" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
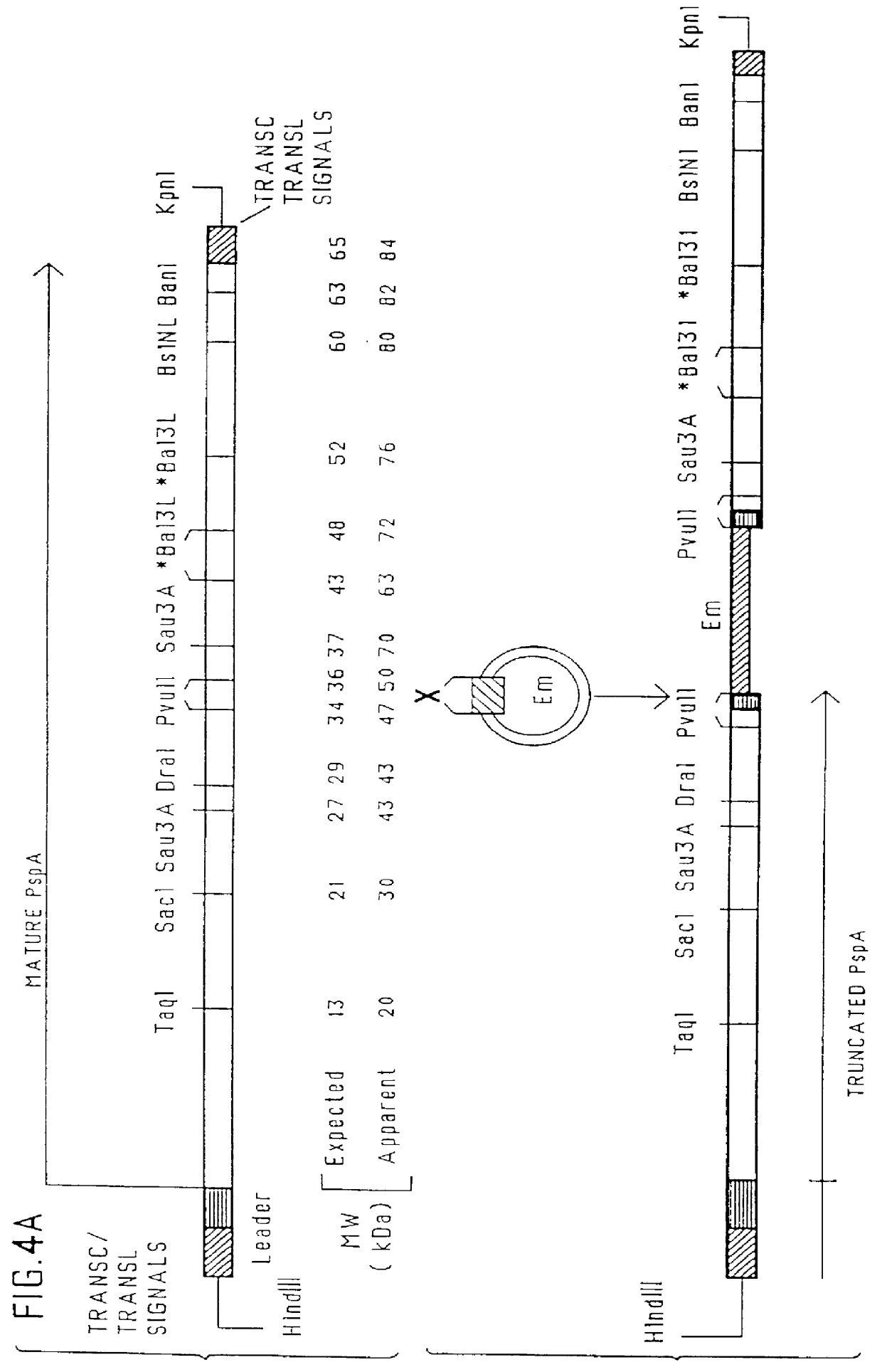
immunogenic compositions for mucosal administration of pneumococcal surface protein A (PspA)
InactiveUS6042838ALittle or no tropism for the GALTGood mucosal immunogenAntibacterial agentsBacterial antigen ingredientsCoccidiaPneumococcal surface protein A
Mucosal administration, particularly intranasally, of killed whole pneumococci, lysate of pneumococci and isolated and purified PspA, as well as immunogenic fragments thereof, particularly when administered with cholera toxin B subunit, provides protection in animals against pneumococcal colonization and systemic infection. The ability to elicit protection against pneumococcal colonization in a host prevents carriage among immunized individuals, which can lead to elimination of disease from the population as a whole.
Owner:UNIVERSITY OF ALABAMA
Mucosal administration of pneumococcal antigens
InactiveUS6027734ALittle or no tropism for the GALTGood mucosal immunogenAntibacterial agentsBiocideDiseasePneumococcal antigen
Mucosal administration, particularly intranasally, of killed whole pneumococci, lysate of pneumococci and isolated and purified PspA, as well as immunogenic fragments thereof, particularly when administered with cholera toxin B subunit, provides protection in animals against pneumococcal colonization and systemic infection. The ability to elicit protection against pneumococcal colonization in a host prevents carriage among immunized individuals, which can lead to elimination of disease from the population as a whole.
Owner:UAB RES FOUND
Pharmaceutical Proteins, Human Therapeutics, Human Serum Albumin Insulin, Native Cholera Toxin B Subunit on Transgenic Plastids
InactiveUS20110179530A1Eliminate needLarge biomassBryophytesMicroorganismsOral tolerizationInsulin-like growth factor
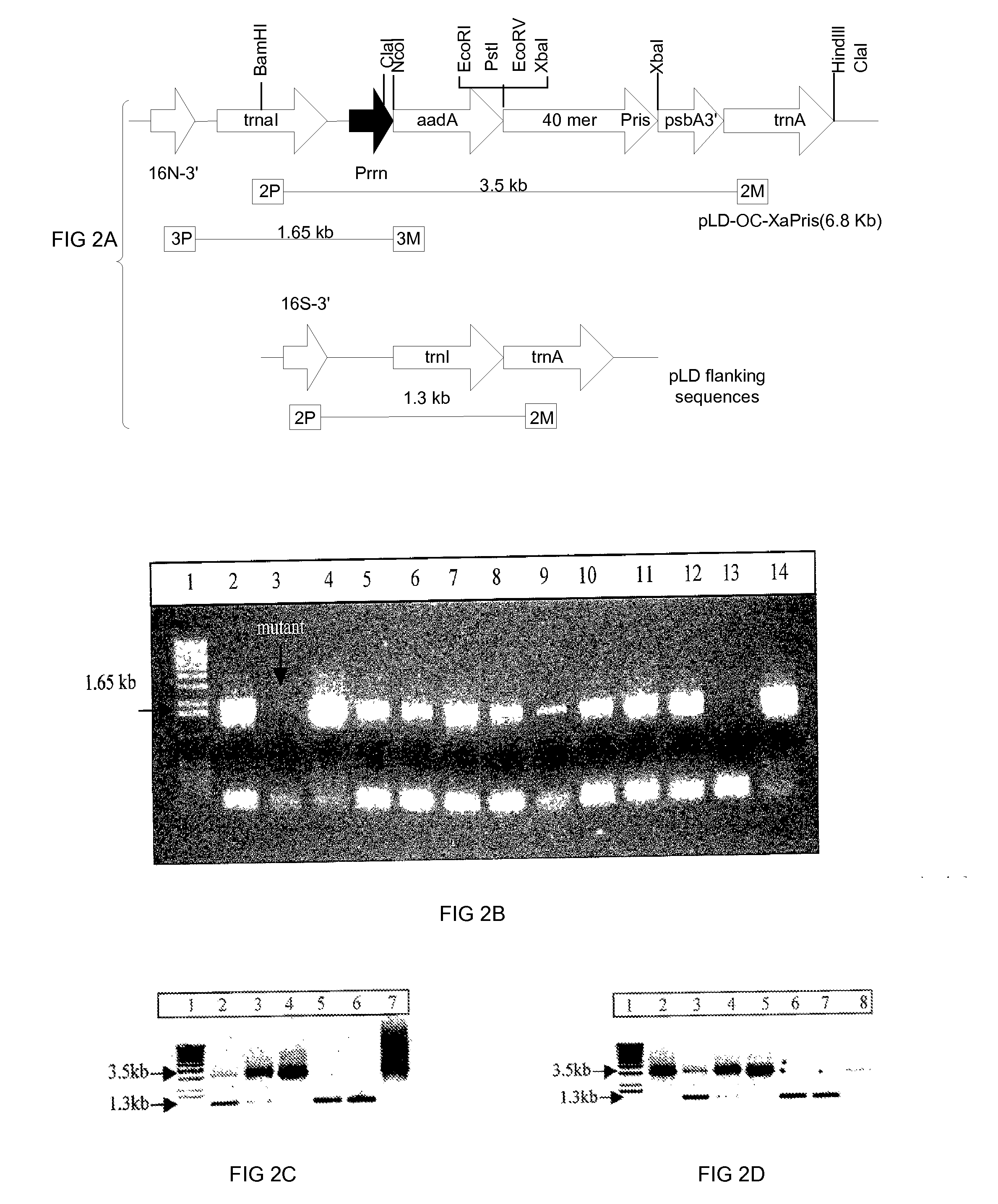
This invention relates in part to synthesizing high value pharmaceutical proteins in transgenic plants by chloroplast expression for pharmaceutical protein production. We use poly(GVGVP), for example, as a fusion protein to enable hyper-expression of insulin and to accomplish rapid one step purification of fusion peptides utilizing the inverse temperature transition properties of this polymer. We also use insulin-CTB fusion protein in chloroplasts of nicotine free edible tobacco (LAMD 605) for oral delivery. This invention includes expression of native cholera toxin B subunit gene as oligomers in transgenic tobacco chloroplasts which may be utilized in connection with large-scale production of purified CTB, as well as an edible vaccine if expressed in an edible plant, as a transmucosal carrier of peptides to which it is fused to enhance mucosal immunity, and / or to induce oral tolerance of the products of these peptides. The present invention also relates in part to recombinant DNA vectors for enhanced expression of human serum albumin, insulin-like growth factor I, and interferon-α 2 and 5, via chloroplast genomes.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Helicobacter pylori multivalent epitope vaccine and preparation method thereof
ActiveCN105169381ASmall molecular weightBiological toxicity avoidanceBacteriaDigestive systemEscherichia coliBiology
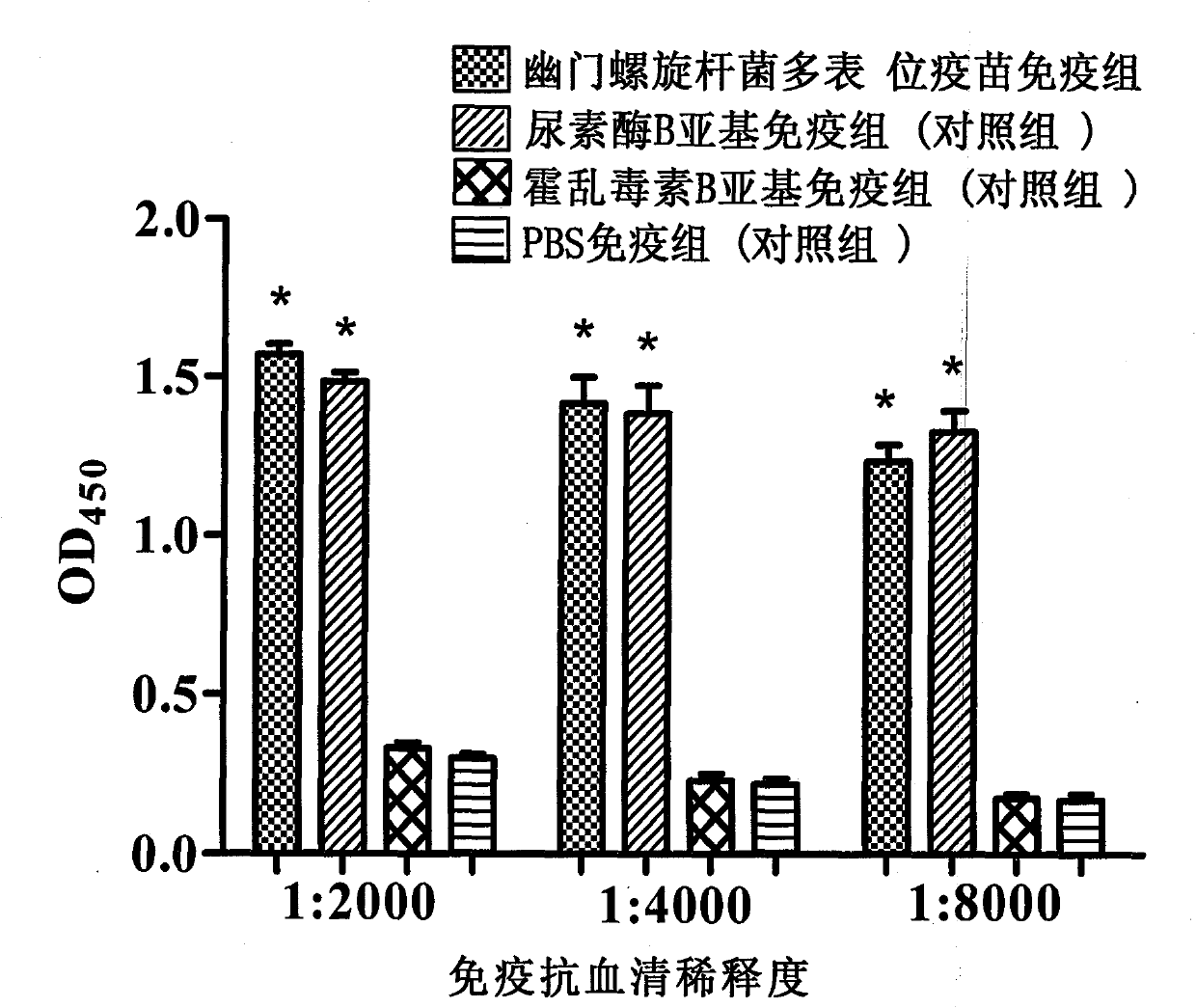
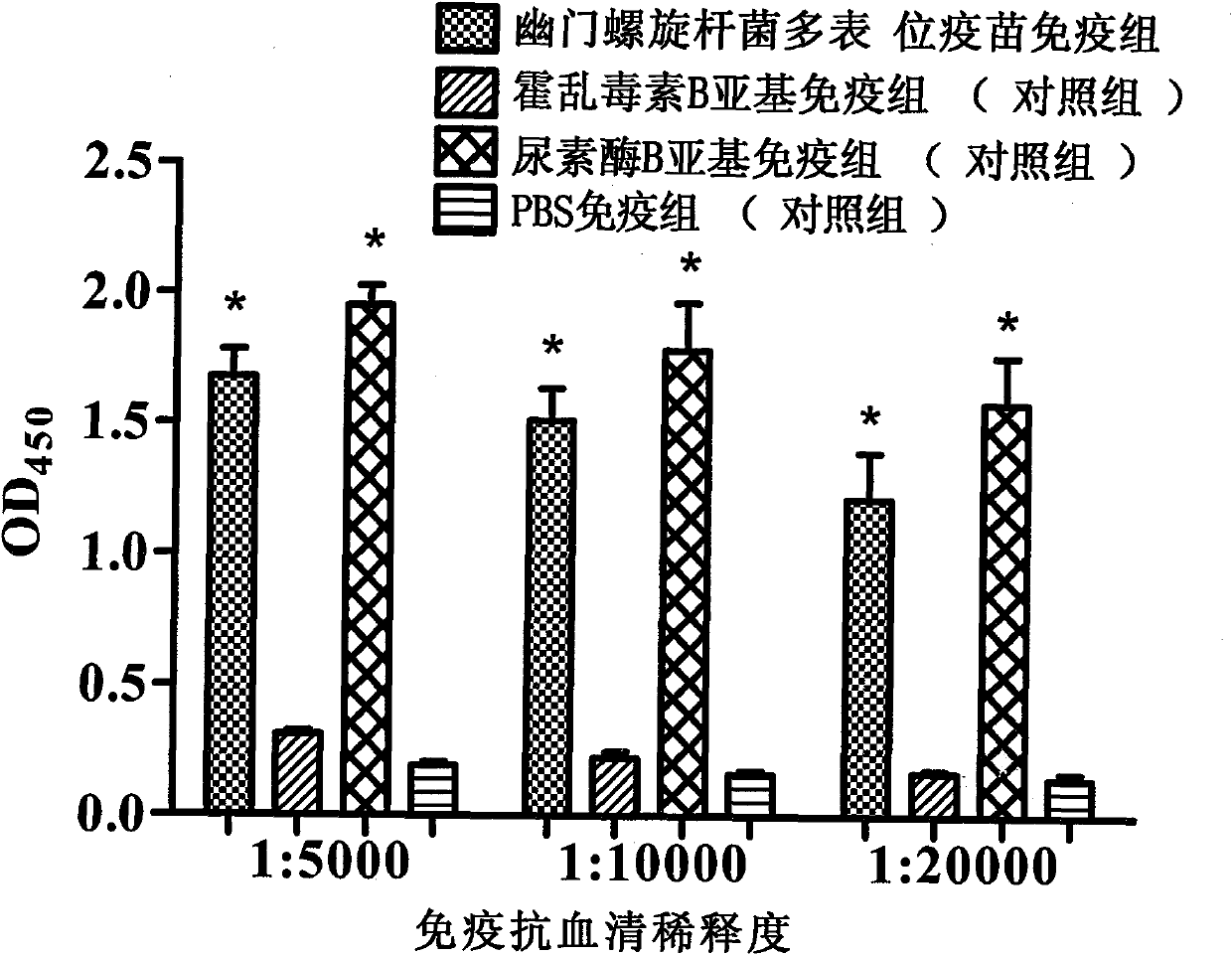
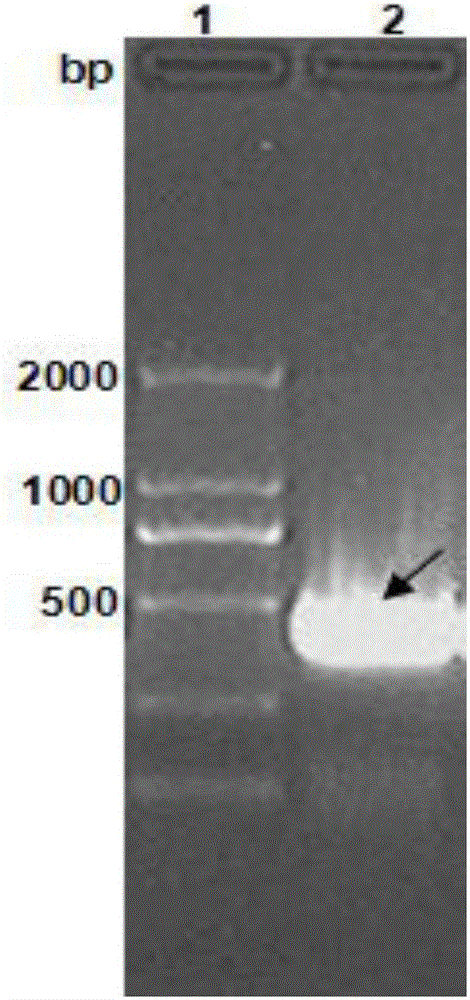
The present invention provides a Helicobacter pylori multivalent epitope vaccine, wherein the activity is a polypeptide, and the polypeptide comprises urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment, neutrophil activating protein NAP and cholera toxin subunit B. According to the present invention, the artificial gene is synthesized through the gene synthesis technology, and comprises the gene sequences of urease subunit A, urease subunit B, adhesin HpaA, heat shock protein HSP60 advantage Th, B cell epitope or segment and neutrophil activating protein NAP, the artificial gene is coupled to cholera toxin subunit B gene to form a fusion gene, the fusion gene is expressed through escherichia coli, and protein purification is performed to obtain the multivalent epitope vaccine; and the multivalent epitope vaccine can stimulate the body to produce the T cell immune response and the antibody humoral immunoresponse against urease, adhesin HpaA, heat shock protein HSP60 and neutrophil activating protein NAP, and can be used for prevention and treatment of Helicobacter pylori infection-related diseases.
Owner:NINGXIA MEDICAL UNIV
Novel helicobacter pylori multiepitope vaccine and preparation method thereof
InactiveCN102178941ANovel structureImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseEscherichia coli
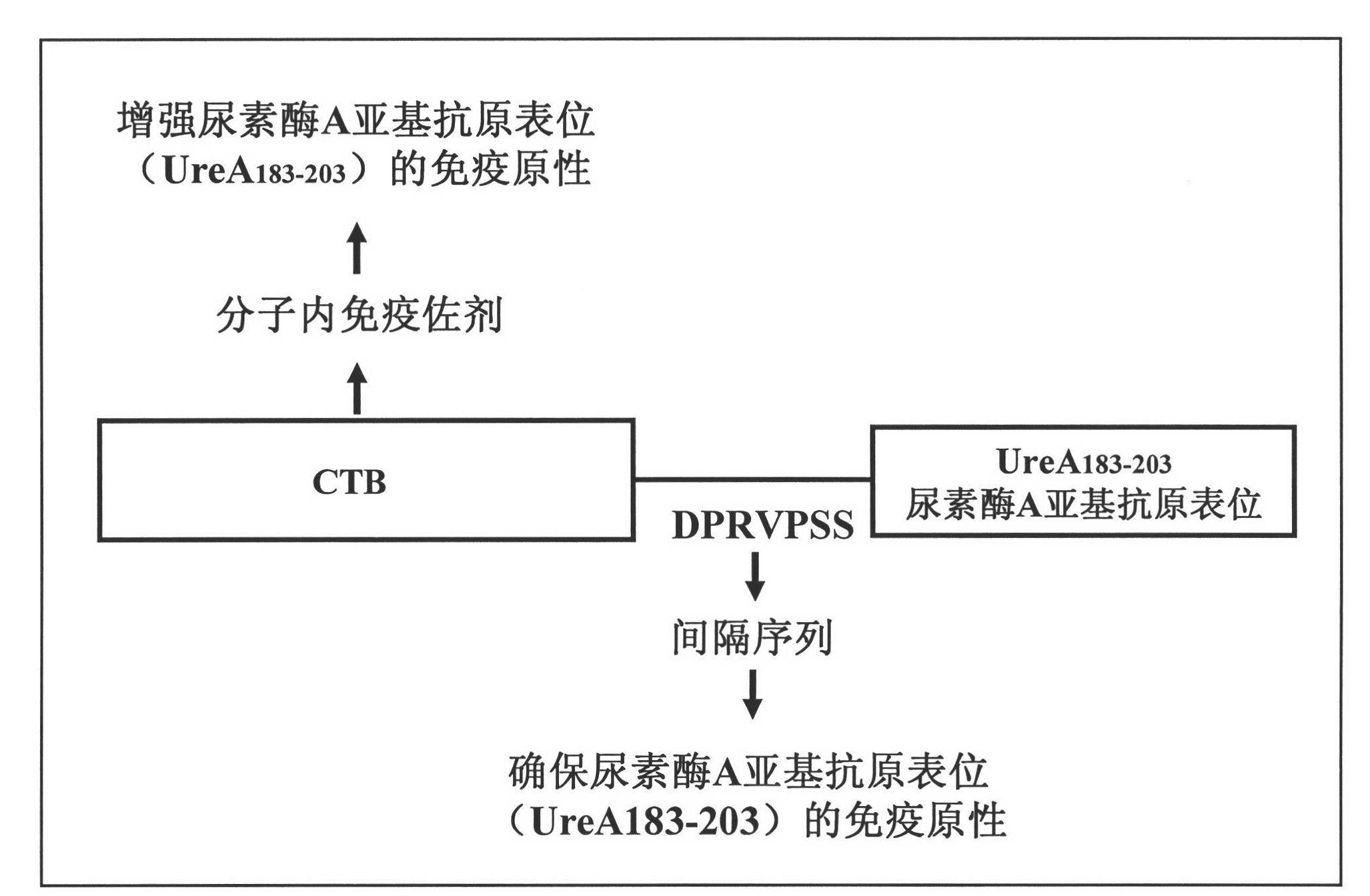
The invention provides a helicobacter pylori multiepitope vaccine. The active ingredient of the helicobacter pylori multiepitope vaccine is a piece of polypeptide; and the helicobacter pylori multiepitope vaccine mainly comprises a multi-copy body of Th and B cell antigen epitopes of helicobacter pylori urease A and B bi-subunit and a mucosal immune adjuvant of a cholera toxin B subunit. The preparation method mainly comprises the following steps of: synthesizing an artificial gene by using a gene synthesis technology, wherein the artificial gene comprises a gene sequence of the multi-copy body of the Th and B cell antigen epitopes of the helicobacter pylori urease A and B bi-subunit; coupling the artificial gene with the gene sequence of the cholera toxin B subunit to form a fusion gene;and expressing the fusion gene by using an escherichia coli prokaryotic expression system, and performing protein purification to obtain the helicobacter pylori multiepitope vaccine. The helicobacterpylori multiepitope vaccine can induce an organism to generate T cell immune response and high-titred specific antibody humoral immune response aiming at the urease A and B bi-subunit, and can be used for preventing and treating helicobacter pylori infection related diseases.
Owner:CHINA PHARM UNIV
A kind of bacillus amyloliquefaciens wh3 and its preparation method and application
InactiveCN102286408AOral lowLow injection toxicityBacteriaMicroorganism based processesFreund adjuvantSclerotinia
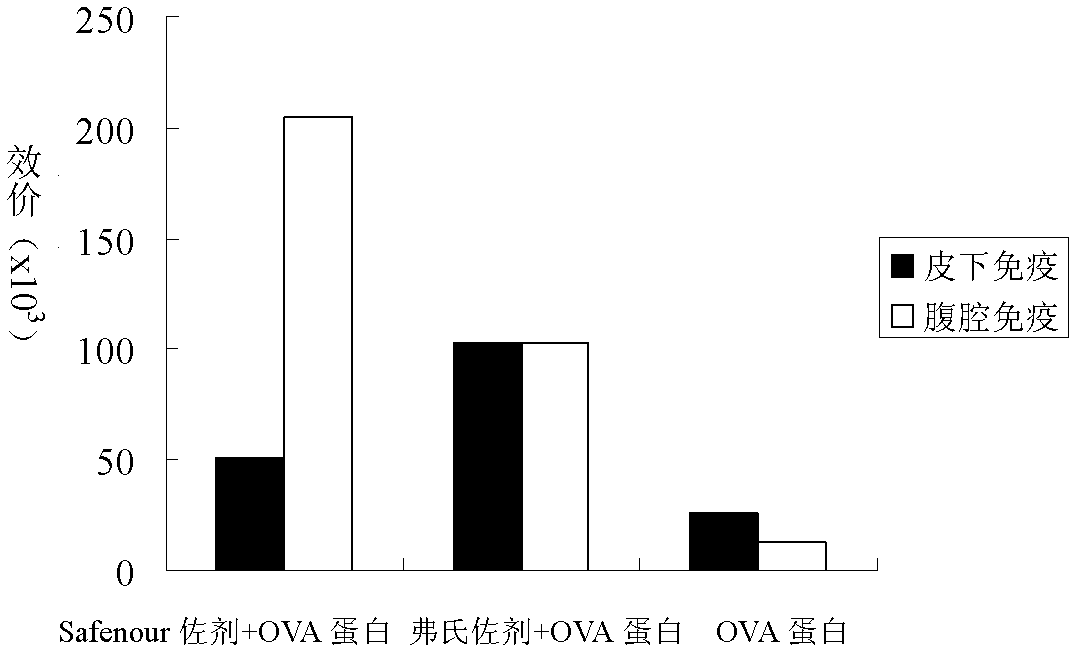
The invention discloses a Bacillus amyloliquefaciens WH3 strain, and a preparation method and application thereof. The preparation method comprises the following steps: 1, separation and identification of bacteria: separating bacteria resistant to rape sclerotinia rot from rape seedlings, and carrying out 16SrDNA and morphological identification to determine that the WH3 strain obtained by separation is Bacillus amyloliquefaciens; 2, separation, purification and identification of an antifungal active substance Safenour: fermenting the WH3 strain, extracting the antifungal active substance, separating and purifying through a sephadex column, and carrying out MALDI-TOF (matrix-assisted laser desorption / ionization-time of flight ) mass spectrometry on the active antifungal substance to inferthat the substance is a ring type polypeptide; and 3, application of the strain in the preparation of vaccines and immunological adjuvants. After the Safenour used as the adjuvant is mixed with a protein antigen and mice are respectively immunized through oral administration and injection of the mixture, effective body fluid and cellular immune response can be activated, and a high-titer specificantibody can be detected in the blood serum. The Safenour has low production cost and high stability, does not need to be emulsified when mixed with the antigen, and has a better immunoenhancement effect in comparison with Freund adjuvants and cholera toxin B subunits.
Owner:武汉光谷世傲生物科技有限公司
Multimeric protein having effect of brain targeting, and preparation method and usage thereof
InactiveCN104387472AIncrease contentRich research methodsNervous disorderPeptide/protein ingredientsEscherichia coliKanamycin
The invention discloses a multimeric protein having the effect of brain targeting, and a preparation method and usage thereof. The method comprises the steps: firstly, expressing cholera toxin B subunit and a fusion protein EGFP-CTA2-TAT of three proteins: an enhanced green fluorescent protein, a cholera toxin A subunit and cell-penetrating peptide in escherichia coli by incompatible double plasmid systems to obtain CTB gene by PCR amplification; cloning the gene segment into a carrier pET-28a to obtain a recombinant plasmid pET-28a-CTB; using wild type CTA2, EGFP and TAT amino acid sequences as templates, inserting 3 enzyme cutting sites and linkers between EGFP and CTA2, and optimizing to obtain codons suitable for expression in escherichia coli; cloning the gene segment into a carrier PET-22b (+) to obtain recombinant plasmid PET-22b-EGFP-CTA2-TAT; using different resistances of PET-28a-CTB and PET-22b-EGFP-CTA2-TAT, and co-transforming the different resistances of the PET-28a-CTB and the PET-22b-EGFP-CTA2-TAT into escherichia coli BL21, to obtain engineering bacteria after screening under double resistance selection pressure of penbritin and kanamycin. CTB5 / EGFP-CTA2-TAT chimeric proteins can be obtained after inducible expression of the engineering bacteria by IPTG. The invention further discloses the preparation method and usage of the protein.
Owner:GUANGDONG UNIV OF TECH
Carrier protein of bacterial polysaccharide conjugate vaccine and application thereof
ActiveCN106511994AUniform polysaccharide binding siteQuality controllableAntibacterial agentsImmunoglobulins against bacteriaConjugate vaccineCarrier protein
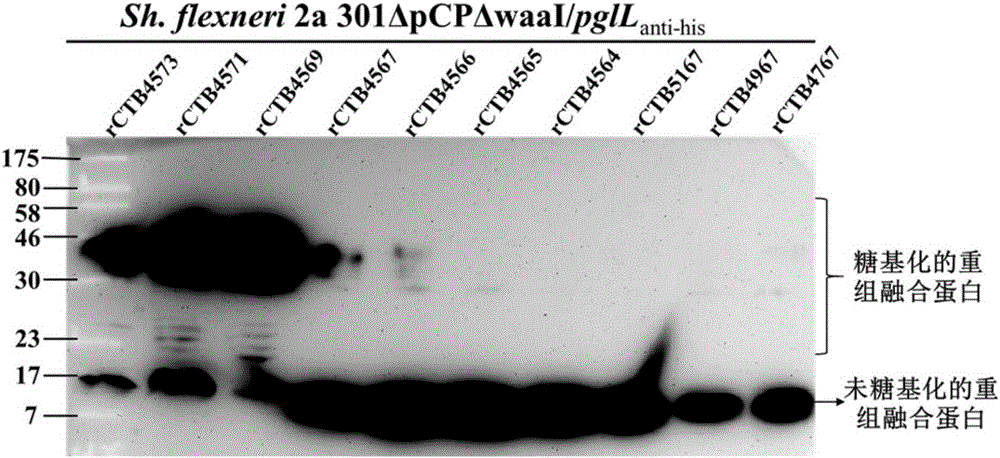
The invention discloses a bacterial polysaccharide O-glycosylation modified recombinant cholera toxin B subunit fusion protein and an application thereof. The present invention provides a conjugate of polysaccharide and protein which is coupled by the bacterial polysaccharide and recombinant cholera toxin B subunit fusion protein. The bacterial polysaccharide is connected with the glycosylation sites of the recombinant cholera toxin B subunit fusion protein in the form of O-glucosidic bond. The experiment indicates that the preparation of the bacterial polysaccharide protein conjugate vaccine from O-glycosylation modified recombinant cholera toxin B subunit fusion protein can enhance the ability of inducing animals to generate anti-polysaccharide antibodies, avoid miscellaneous problems of pathogen culture, enhance the vaccine homogeneity and production efficiency, and reduce the vaccine preparation cost, therefore possessing wide application prospect.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Meningococcus capsular polysaccharide polyvalent multivalent conjugate vaccine, preparation method and application thereof
InactiveCN102078604AHigh yieldHigh purityNitro compound active ingredientsCarrier-bound antigen/hapten ingredientsFlocculationConjugate vaccine
The invention discloses a meningococcus capsular polysaccharide polyvalent multivalent conjugate vaccine, a preparation method and application thereof. The polyvalent multivalent conjugate vaccine comprises at least two conjugates of group A, group C, group Y or Group W135 meningococcus capsular polysaccharide and cholera toxin B-subunit. The invention improves the method for extracting the meningococcus capsular polysaccharide, and improves the yield and purity of the capsular polysaccharide by adopting a flocculation extraction method under an electric field.
Owner:BEIJING MINHAI BIOTECH +1
Helicobacter pylori epitope vaccine, design method thereof, preparation method thereof and application thereof
InactiveCN102151332AAntibacterial agentsAntibody medical ingredientsEscherichia coliSpecific antibody
The invention provides a helicobacter pylori epitope vaccine. The active constituent of the helicobacter pylori epitope vaccine is a polypeptide and mainly consists of a cholera toxin B subunit and a B cell antigen epitope from a urease A subunit. A preparation method of the helicobacter pylori epitope vaccine comprises the following steps of: synthesizing the nucleotide sequence of the B cell antigen epitope from the urease A subunit by a PCR (polymerase chain reaction) technology, coupling the nucleotide sequence with the gene sequence of the cholera toxin B subunit to form into a fusion gene, and expressing the fusion gene in the escherichia coli by an expression vector to obtain the fusion protein of the epitope vaccine by means of protein purification. The helicobacter pylori epitope vaccine can be used for inducing the human body to generate an epitope specific antibody which is higher in titer to the urease. In the biologic medical field, the epitope vaccine can be used for preventing and curing the relevant diseases caused by the helicobacter pylori infection, thereby being great in economic benefit and social benefit.
Owner:CHINA PHARM UNIV
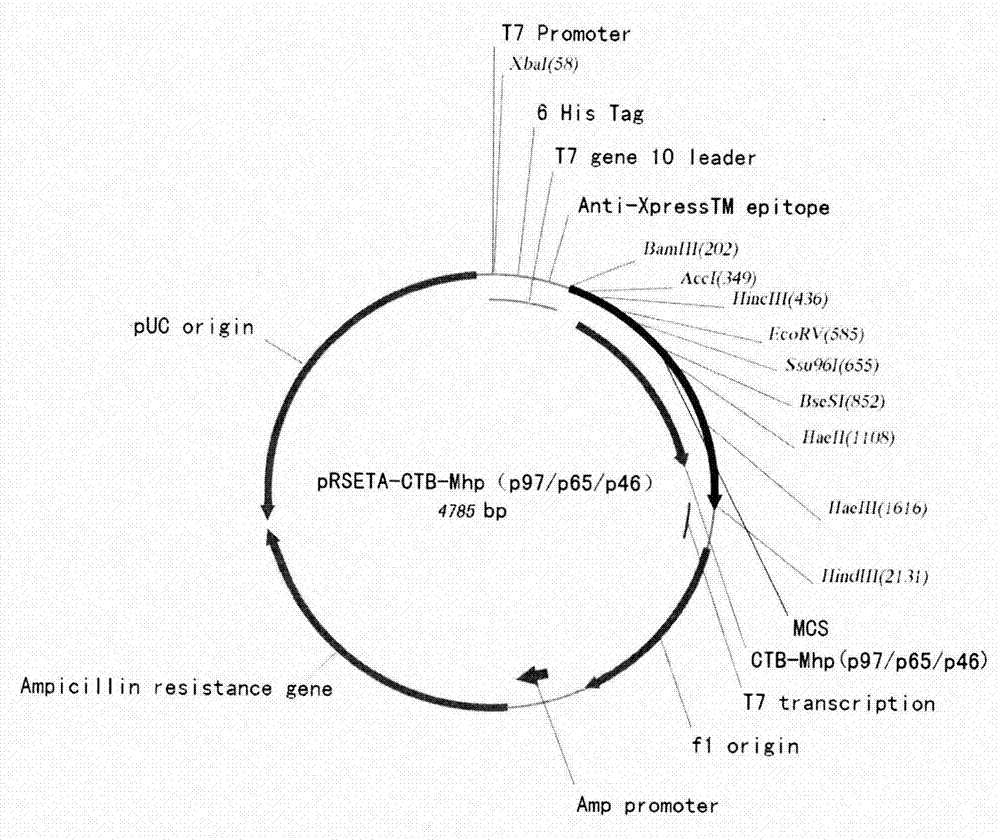
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Expression of recombination SARS virus gene in pleiomorphic Hansen yeast and its use
InactiveCN1475571AAvoid maladaptiveNon-tumorigenicOrganic active ingredientsGenetic material ingredientsAntigen epitopeAntigen
An expression of recombinant SARS virus gene in polymorphic Hanson yeast and its application are disclosed. In the procedure of industrially preparing the expressed product of SARS virus' genes S, S1and S2 and primary antigen epitope gene, the SARS virus' genes S, S1 and S2 and primary antigen epitope gene are redesigned according to the application method of the codon of high-expression gene for Hanson yeast. The high-expression product can be used as vaccine to prevent the atypical pneumatonitis caused by SARS virus. The designed gene can also be used for fusion expression with cholera toxin B subunit gene in Hanson yeast.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Vaccine for blocking transmission of echinococcosis pathogeny echinococcus granulosus from source
ActiveCN107510841AIncreased serum titerImprove immunityBacterial antigen ingredientsProtozoa antigen ingredientsMucosal Immune ResponsesMycobacterium smegmatis
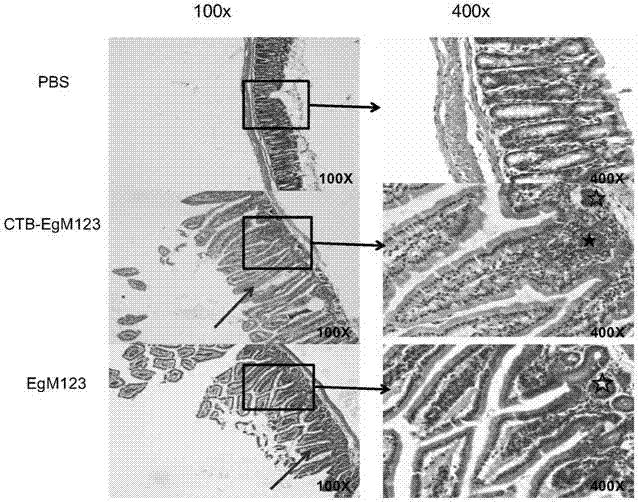
The invention discloses a vaccine for blocking transmission of echinococcosis pathogeny echinococcus granulosus from a source. The vaccine provided by the invention is a CTB-EgM123 vaccine or a complete set of vaccine consisting of the CTB-EgM123 vaccine and a subunit vaccine of an EgM123 protein recombinant mycobacterium smegmatis vaccine. An active ingredient of the CTB-EgM123 vaccine is a fusion protein formed by fusion of a cholera toxin B subunit and an echinococcus granulosus EgM123 protein; the active ingredient of the EgM123 protein recombinant mycobacterium smegmatis vaccine can express the recombinant mycobacterium smegmatis of the echinococcus granulosus EgM123 protein. The vaccine provided by the invention can stimulate the immune response in a host, improves the immune level of the host, enhances the intestinal mucosal immune response, and plays an important protective role in resisting infection of echinococcosis.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
Transformant for blocking effect of porcine somatostatin by oral immunization and application thereof
InactiveCN101892189AImprove securityEnhance immune responseBacteriaBacteria material medical ingredientsCell wallImmunoenhancing factor
The invention discloses transformant. The transformant comprises a recipient bacterium and a recombinant vector transferred to the recipient bacterium, wherein the recipient bacterium is lactococcus lactis NZ9800; the original vector of the recombinant vector is pNZ8112; and the exogenous target gene of the recombinant vector comprises a porcine somatostatin gene, a cholera toxin B subunit gene and a cell wall anchor stator M6 gene. The transformant can enter the porcine intestinal tract by oral administration; compared with injection immunization, the oral immunization mode reduces the toxicity caused by direct contact with a circulatory system and increases the safety of vaccines; and the immune response of organisms is enhanced and the immune effect is improved by combined immunization of the transformant, an antigen gene and an immune enhancing factor.
Owner:ZHEJIANG UNIV
Preparation method of cholera toxin B sub unit
InactiveCN1427010AEnhance the effect of oral leukocyte productionGood effectAntibacterial agentsPeptide/protein ingredientsAntigenMicroorganism
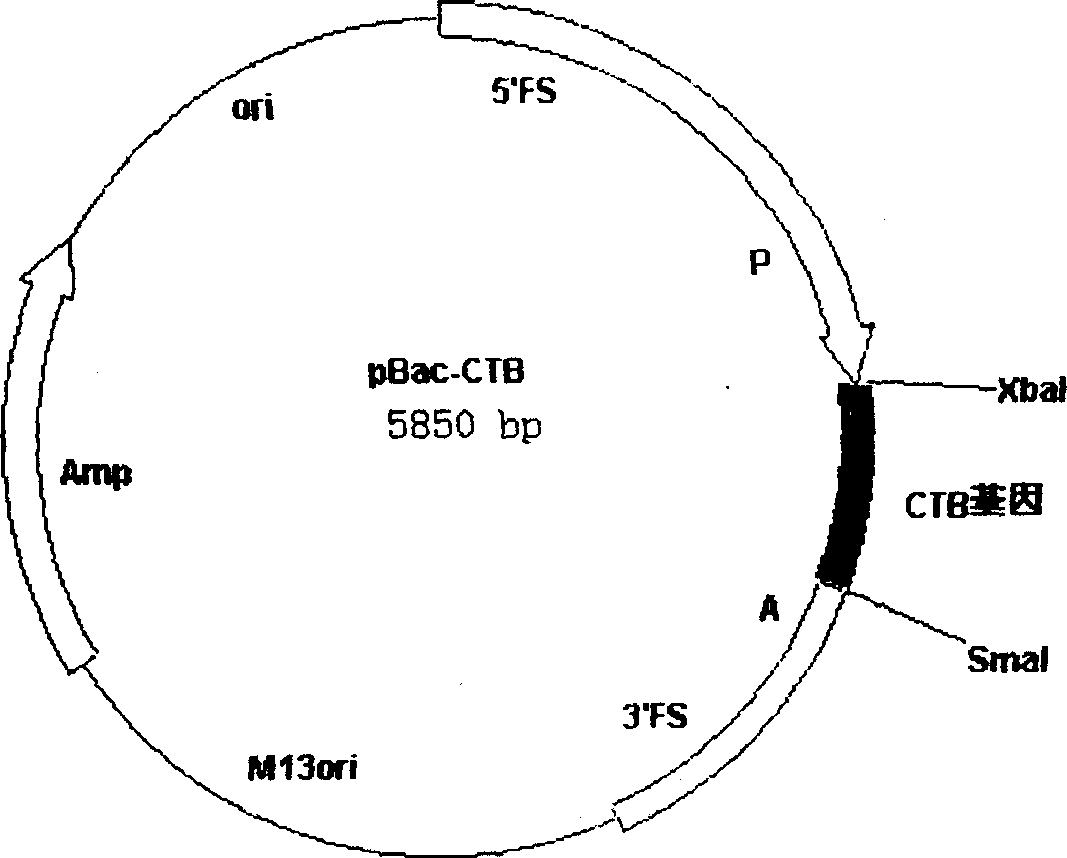
A process for preparing cholera toxin B subunit features that by the expression system of Bombyx mori nuclear polyhedrosis virus, the virus with CTB gene is recombined to obtain the recombinant virus (CGMCC No.0594) which has been preserved by Ordinory Microbe Preservation Center of China Microbial Strain Preservation and Management Committee. An oral medicine for treating cholera is prepared through inoculating the virus to the larva or pupa of Bombyx mori, expressing, separating, purifying, and freeze drying. Its advantages are high curative effect, and low cost.
Owner:ZHEJIANG UNIV
Chloroplast-derived human vaccine antigens against malaria
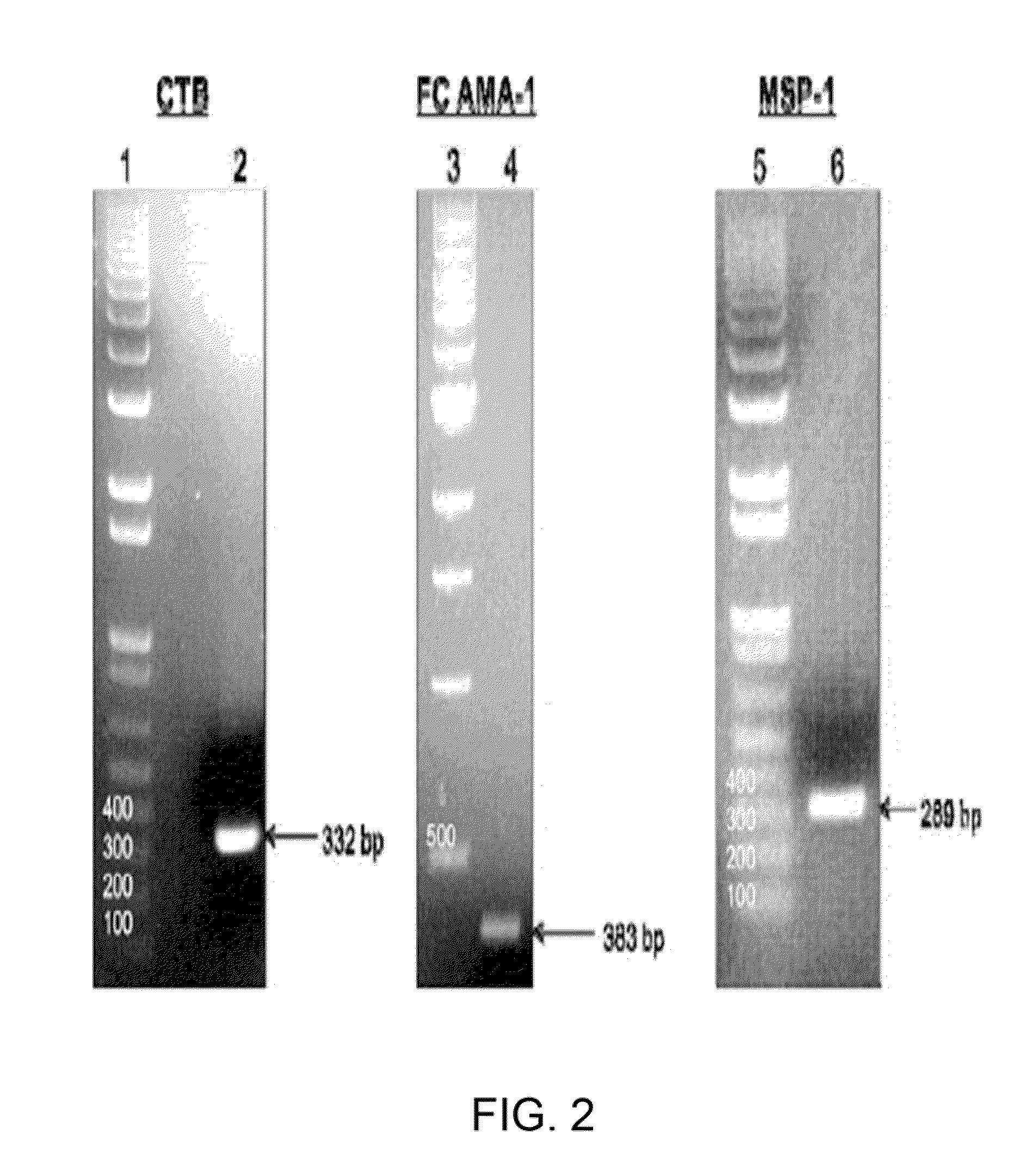
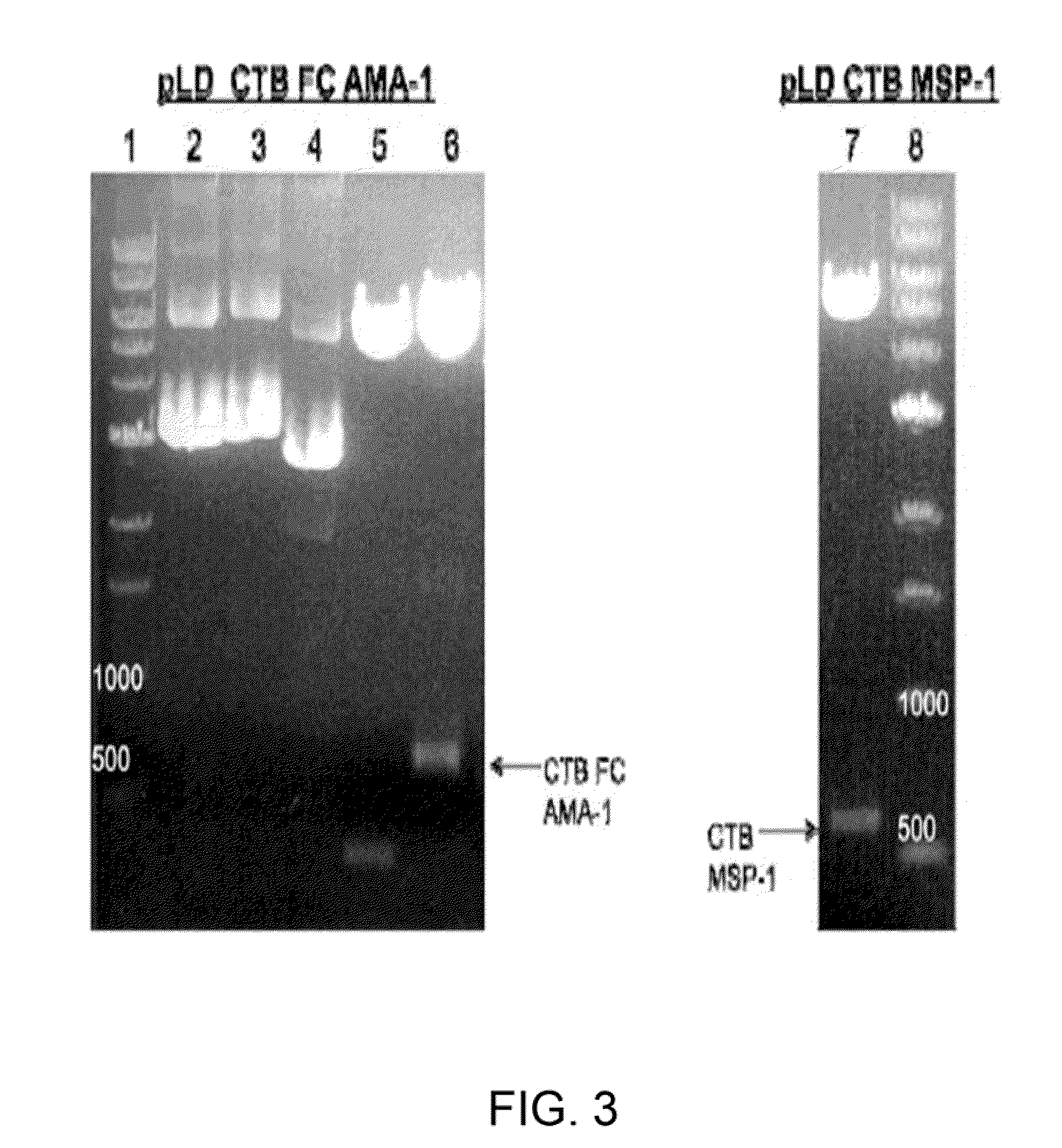
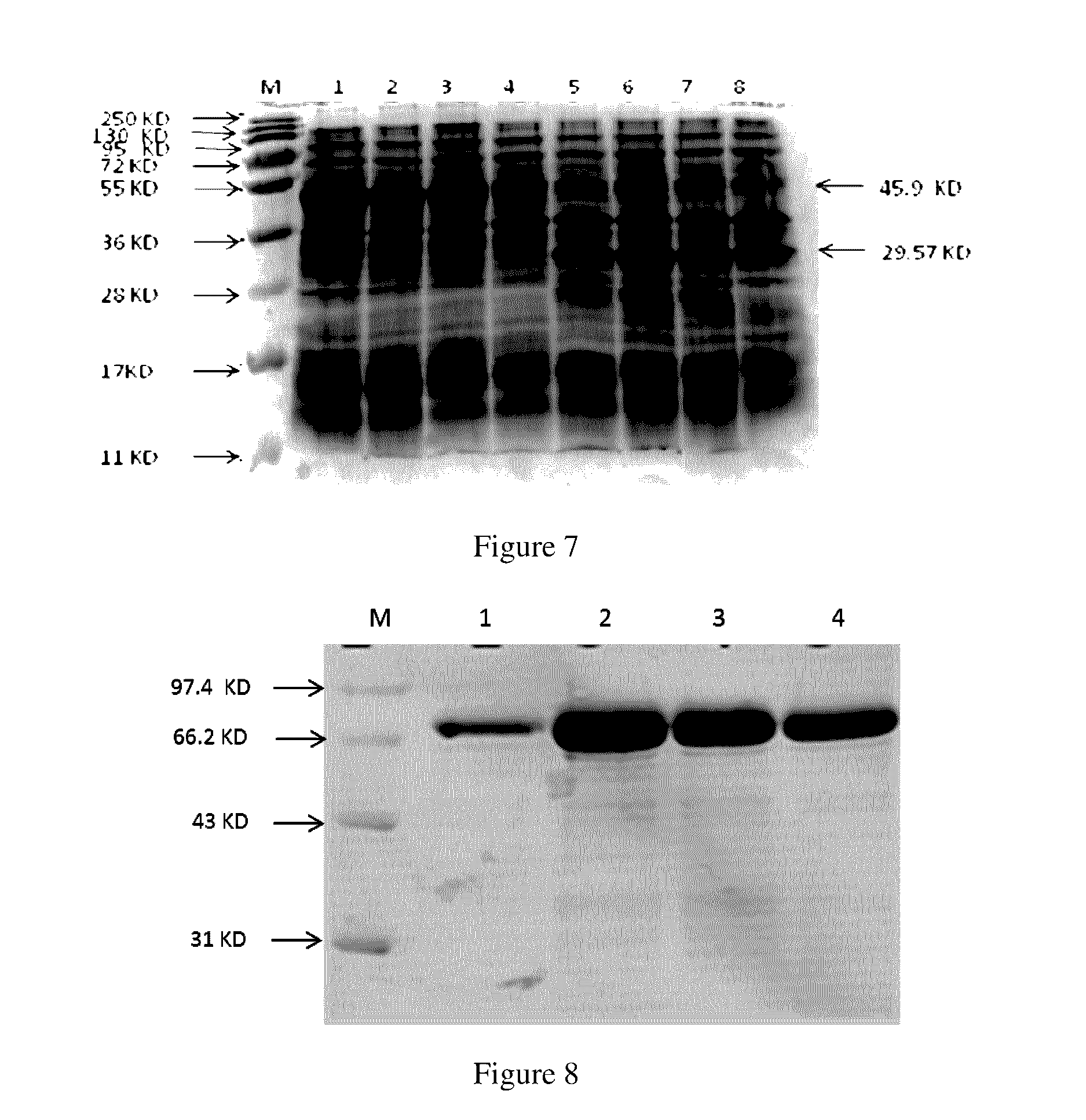
InactiveUS20090297550A1Low costImprove expression levelVaccinesImmunoglobulinsVaccine antigenMalaria
Disclosed is a method of making a malaria vaccine, the method comprising stably transforming a plant by inserting into its plastid genome a nucleic acid sequence encoding and operable to constitutively express a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both; harvesting the stably transformed plant in whole or in part; purifying the expressed malaria antigenic polypeptide from the harvested plant; and packaging the purified antigenic polypeptide under sterile conditions in an amount for a predetermined dosage. Also disclosed is an oral vaccine effective in raising malaria antibodies in a susceptible host, the vaccine comprising leaf material from an edible plant containing plastids stably transformed to constitutively express a fusion polypeptide consisting essentially of cholera toxin B subunit and a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both.
Owner:DANIELL HENRY +1
Helicobacter pylori tetravalent adhesion multi-epitope vaccine and preparation method thereof
ActiveCN105126093ABiological toxicity avoidanceProtect against immunopathological damageAntibacterial agentsPeptide preparation methodsEscherichia coliDisease
The invention provides a multi-epitope vaccine for a helicobacter pylori tetravalent adhesion, wherein the activity of the multi-epitope vaccine is presented as a polypeptide which mainly consists of urease A and B subunits, superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL) as well as cholera toxin B subunit. According to the invention, an artificial gene is synthesized by virtue of gene synthesis technology, wherein the synthesized artificial gene consists of urease A and B subunits, and superior Th and B cell epitopes or fragments of three outer membrane proteins (Lpp20, HpaA and CagL), and the artificial gene is coupled with gene sequence of the cholera toxin B subunit, so as to form a fusion gene. The fusion gene is expressed by escherichia coli, and upon protein purification, the tetravalent adhesion multi-epitope vaccine is obtained. The vaccine can be used for inducing a body to generate T cellullar immunologic response and specific antibody humoral immune response in accordance with the urease A and B subunits and the three outer membrane proteins (Lpp20, HpaA and CagL); and the vaccine is suitable for preventing and controlling helicobacter pylori infection related diseases.
Owner:NINGXIA MEDICAL UNIV
Fusion protein vaccine capable of inhibiting Streptococcus and/or preventing Streptococcus infection
ActiveCN106554421AEasy to removeReduce dosageAntibacterial agentsFungiSide effectStreptococcus infection
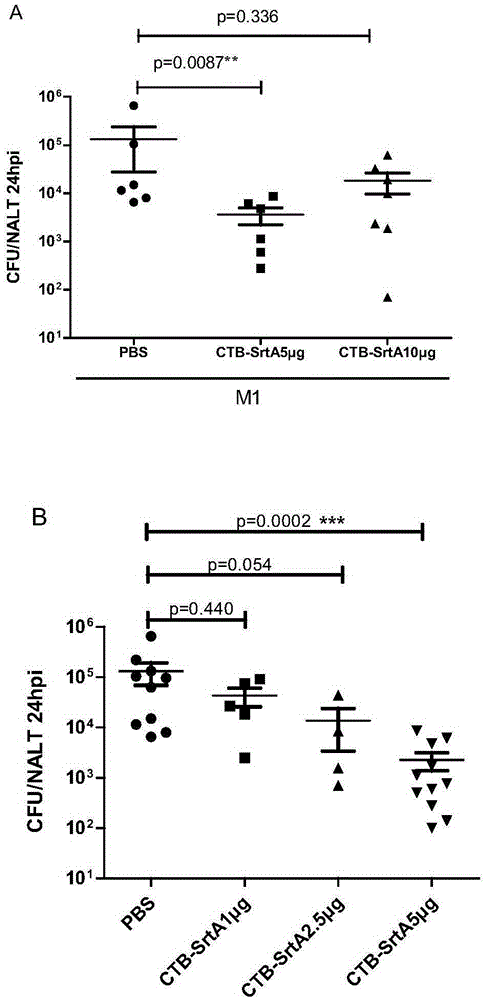
The invention discloses a fusion protein vaccine capable of inhibiting Streptococcus and / or preventing Streptococcus infection. With fusion protein obtained through linkage of sortase A and a cholera toxin B subunit through connecting peptide, the Th17 cell activation level is remarkably increased, the effects of preventing pathogenic bacteria from settling and quickly removing the pathogenic bacteria can be realized, and the fusion protein has protecting effects on Group A Streptococcus of different serotypes and has the superiority of high efficiency, broad spectrum and low cost. With the fusion protein, the use amount of immunogen is reduced, the production technology is simplified, and the production cost is reduced. Meanwhile, the vaccine adopts the way of mucosal immunity, has the characteristics of being free of tissue damage, free of local side effects and convenient to use, and is easy to popularize and use.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Asia 1-type FMDV compound multiepitope
InactiveCN101838319AReduce interactionGood antigenicityVirus peptidesAntiviralsPeptide vaccineGenetic engineering
The invention provides an Asia 1-type FMDV compound multiepitope which adopts an Asia 1-type FMDV capsid protein VP1 antigen activity area as the basic skeleton, and comprises the epitope of Asia 1 / JL / 05 plant VP1, the epitope of the IND 63 / 72 plant VP1, the epitope of YNBS / 58 plant VP1, cholera toxin B subunit and helper T lymphocyte epitope. The compound multiepitope can be used for preparing Asia 1-type FMDV subunit vaccine, nucleic acid vaccine, synthetic peptide vaccine, live vector vaccine and a plurality of genetic engineering vaccines.
Owner:JILIN UNIV
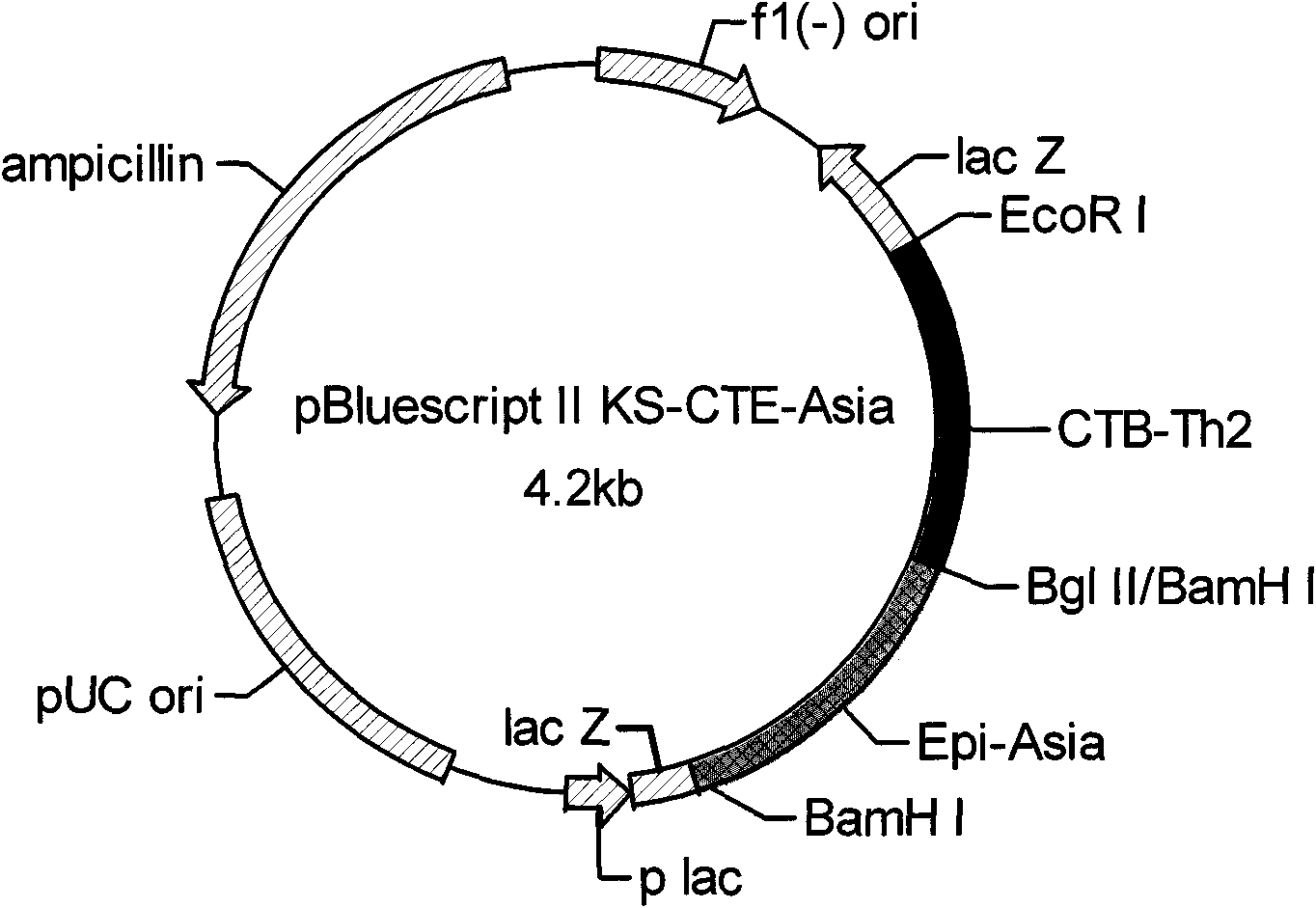
Immunomodulation agent with adjuvant having function for treating atherosclerosis
InactiveCN101036786APeptide/protein ingredientsImmunological disordersAdjuvantNew Zealand white rabbit
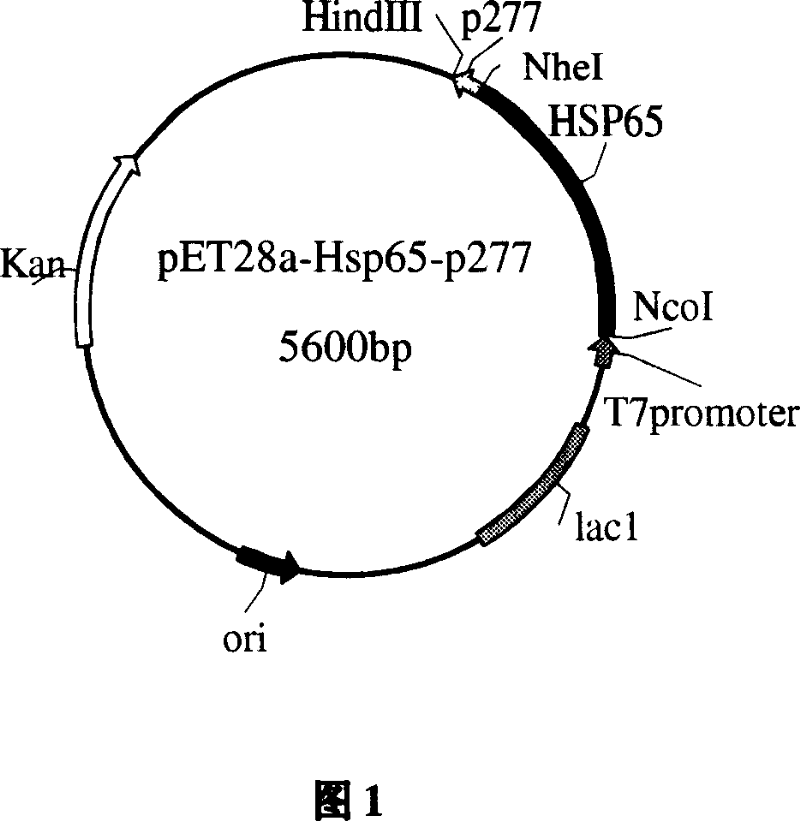
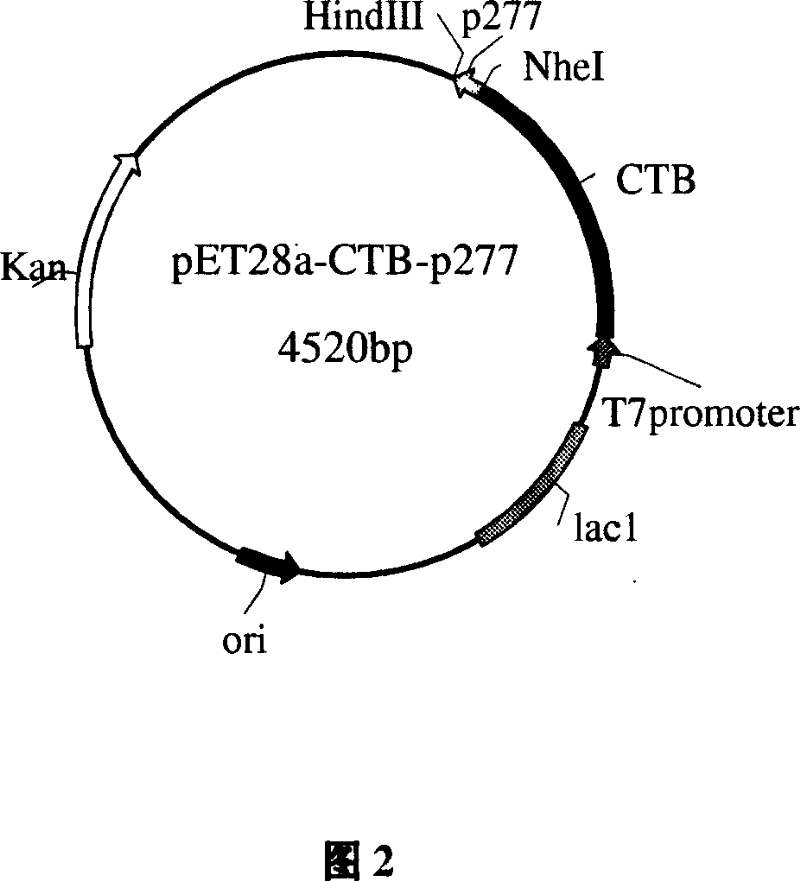
The invention relates to an immune modulator for treating arteriosclerosis. Aiming at antigen peptides's weak immunogenicity, a series of epitopes peptides of antigens (SEQ ID NO.1) originating from heat shock protein (HSP60) are inserted into the downstream of Cholera toxin B subunit(CTB), and fusion expression of antigens epitopes peptides and CTB is realized. Produced fusion protein (CTB-p277) and Heat shock protein 65 (HSP65) are coupled chemically, without adjuvant, and directly used immunization. The coupled protein can stimulate body to produce high titer antibody aiming at P277 through mucosal immunity pathway, obviously inhibit occurrence of New Zealand white rabbits's arteriosclerosis, and can have a function for treating arteriosclerosis.
Owner:CHINA PHARM UNIV
Preparation method of cholera toxin B subunit protein having biological activity
ActiveCN106946984AGood effectHigh protein expressionDepsipeptidesPeptide preparation methodsEscherichia coliFluorescence
The present invention discloses a preparation method of cholera toxin B subunit protein having biological activity. According to the present invention, a lot of cholera toxin subunits are expressed in Escherichia coli by using a prokaryotic expression vector, and then in vitro efficient renaturation is performed to obtain a large number of the cholera toxin B subunit having biological activity; the operations can be performed in the general laboratory, and no pollution is generated; and the most important advantage is that the labeling with various fluorescence, isotopes and tracers can be performed according to the experimental needs.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Design and preparation method and application of novel fasciola hepatica multi-epitope vaccine
The invention provides a novel fasciola hepatica multi-epitope vaccine. An active ingredient of the novel fasciola hepatica multi-epitope vaccine is a polypeptide. The polypeptide is mainly formed by fasciola hepatica sphingolipid activated mucin-like protein-2 Th, a multicopy body of B-cell epitope and a mucosal immunologic adjuvant cholera toxin B subunit. An artificial gene is mainly synthesized through the gene synthesis technology and comprises Th of sphingolipid activated mucin-like protein-2 and a gene sequence of the multicopy body of the B-cell epitope, then the artificial gene is coupled with the gene sequence of the cholera toxin B subunit, and a fusion gene is formed. An escherichia coli prokaryotic expression system is utilized for expressing the fusion gene, and the fasciola hepatica multi-epitope vaccine is obtained after protein purification. The fasciola hepatica multi-epitope vaccine can induce an organism to generate sphingolipid activated protein T cell immune responses and high-titer specific antibody humoral immune responses, and can be used for preventing and treating fasciola hepatica infection related diseases.
Owner:QINGHAI UNIVERSITY
Vibrio cholerae O139 capsular polysaccharide conjugate vaccine and preparation method thereof
The invention discloses a vibrio cholerae O139 capsular polysaccharide conjugate vaccine which comprises a conjugate of vibrio cholerae O139 capsular polysaccharide and a protein carrier, wherein the protein carrier is preferential to be a cholera toxin B-subunit. The invention also provides a preparation method of the conjugate vaccine, which comprises the step of coupling the vibrio cholerae O139 capsular polysaccharide and the carrier protein with a mass ratio of (0.2-3):1. After the vibrio cholerae O139 capsular polysaccharide conjugate vaccine is subcutaneously injected to an immune mouse, the conjugate vaccine can excite the mouse to generate antibodies for resisting vibrio cholerae O139 and antibodies for resisting cholera toxin, thus the titer of vibriocidal antibodies can be improved by more than 2 times, and the titer of the antibodies for resisting the cholera toxin (IgG and IgA) can be improved by more than 4 times.
Owner:BEIJING MINHAI BIOTECH
Expression system for the b subunit of cholera toxin
The present invention provides an expression system for obtaining improved cholera toxin subunit B (CTB) production, wherein the expression system comprises a Vibrio cholerae host cell lacking thyA gene function; and an expression vector comprising a functional thyA gene and a CTB gene , the CTB gene does not substantially contain the flanking sequences immediately adjacent to the 5' and 3' ends of the CTB gene in the native genome of the host cell as the source of the CTB gene. The present invention also provides methods for producing CTB, and isolated nucleic acid constructs for use as expression vectors in expression systems.
Owner:SBL VACCIN AB
Polysaccharide and protein conjugate
InactiveCN102580073AImprove immunityRaise antibody levelsAntibacterial agentsAntibody medical ingredientsBactericidal antibodyBactericidin
The invention discloses a polysaccharide and protein conjugate. The polysaccharide and protein conjugate is formed by coupling vibrio cholerae lipopolysaccharide and cholera toxin non-toxic subunit protein, wherein the vibrio cholerae lipopolysaccharide is from vibrio cholerae 0139, the protein content in the vibrio cholerae lipopolysaccharide is not more than 2 percent, the nucleic acid content is not more than 5 percent, and the molecular weight of the vibrio cholerae lipopolysaccharide is 8Kd. The immune effect of the polysaccharide and protein conjugate is higher than that of common rBS-WC (recombinant B subunit-killed whole-cell cholera) vaccine, single LPS (lipopolysaccharide) vaccine or single CTB (cholera toxin B-subunit) vaccine, the conjugate can enhance the immunogenicity of LPS to make an induced immunity reaction have immunologic memory and potentiation effect to generate massive bactericidin, is suitable for various kinds of people, and can initiate mucosal immunity of a body to realize IgA (immunoglobulin A) antibodies massively exist in intestinal secretion fluid and bile.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Designing and preparing method and application of novel alveolar echinococcosis subunit vaccine
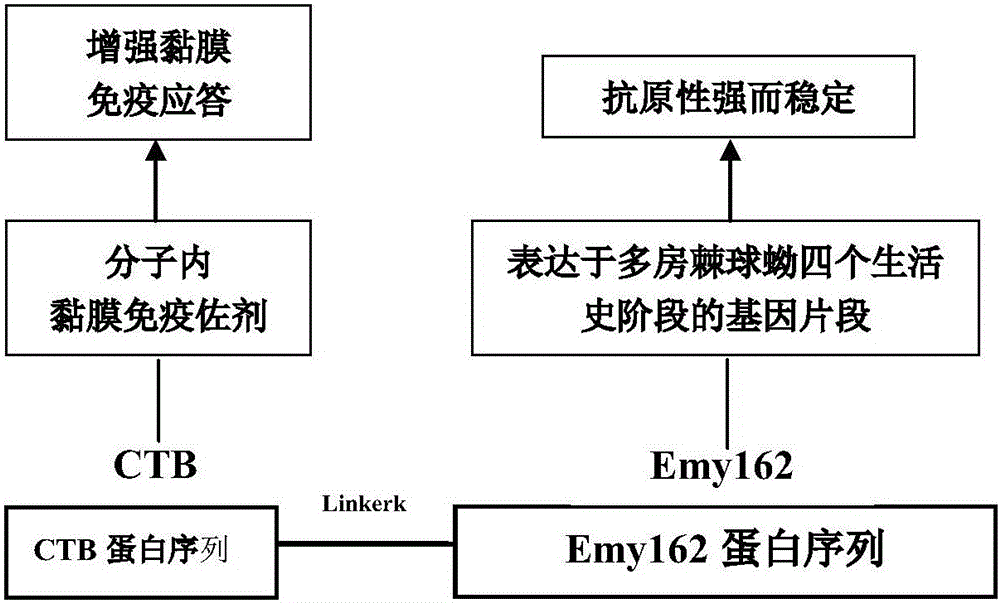
InactiveCN106075416ALow immunogenicityImproving immunogenicityBacteriaNucleic acid vectorEscherichia coliDisease
The invention provides a novel alveolar echinococcosis subunit vaccine. A polypeptide serves as an active ingredient of the alveolar echinococcosis subunit vaccine, which is mainly composed of a surface antigen Emy162 of alveolar echinococcosis and a mucosal immune adjuvant, namely cholera toxin B subunit (CTB). According to the invention, the gene sequence of the alveolar echinococcosis surface antigen Emy162 is synthesized by virtue of a gene synthesis technology, and then the gene is coupled with the gene sequence of the cholera toxin B subunit, so that a fusion gene is formed. The fusion gene is expressed by virtue of an escherichia coli prokaryotic expression system, and protein is purified, so that the alveolar echinococcosis subunit vaccine is obtained. The alveolar echinococcosis subunit vaccine is capable of inducing a body to generate alveolar echinococcosis targeted T cell and B cell immune response and high-titer specific antibody humoral immune response, and the subunit vaccine can be used for preventing and treating alveolar echinococcosis infection related diseases.
Owner:QINGHAI UNIVERSITY
Antigen chimera, antigen composition, vaccine, method of preparing the same and cassette thereof
ActiveUS20160368951A1Improving immunogenicityIncrease volumeAntibacterial agentsAntibody mimetics/scaffoldsEscherichia coliPentamer
The present invention provides an antigen chimera, comprising: a fusion protein of an antigen and a mucosal immune adjuvant protein monomer capable of forming a multimer; and the mucosal immune adjuvant protein monomer capable of forming the multimer; wherein the mucosal immune adjuvant protein monomer capable of forming the multimer is one selected from cholera toxin B subunit (CTB) and E. coli heat-labile enterotoxin B subunit (LTB), the multimer is a pentamer, and in the chimera the molar ratio between the fusion protein and the mucosal immune adjuvant protein monomer capable of forming multimers is 1:4. In the present invention, a characteristic that a mucosal immune adjuvant protein can form a pentamer is used to form a chimeric structure, so as to form an antigen having a higher potency. Moreover, a mucosal immune adjuvant protein is used to improve an immune effect, so as to improve an effect of enhancing antigen immunogenicity. In addition, the chimeric protein antigen formed with the recombined antigen of the present invention stimulates a mucous membrane to produce secretory IgA and induce the occurrence of mucosal immunity.
Owner:SHANGHAI UNITED CELL BIOTECH
Chloroplast-derived human vaccine antigens against malaria
Disclosed is a method of making a malaria vaccine, the method comprising stably transforming a plant by inserting into its plastid genome a nucleic acid sequence encoding and operable to constitutively express a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both; harvesting the stably transformed plant in whole or in part; purifying the expressed malaria antigenic polypeptide from the harvested plant; and packaging the purified antigenic polypeptide under sterile conditions in an amount for a predetermined dosage. Also disclosed is an oral vaccine effective in raising malaria antibodies in a susceptible host, the vaccine comprising leaf material from an edible plant containing plastids stably transformed to constitutively express a fusion polypeptide consisting essentially of cholera toxin B subunit and a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
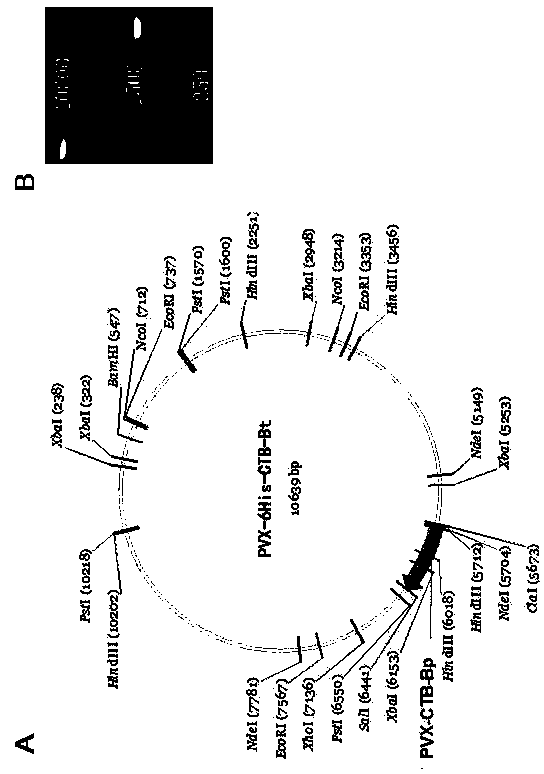
Expression vector PVX-6His-CTBt-Bt for producing multi-epitope vaccine of hepatitis c virus
InactiveCN104353071ADetermining the inhibitory effectAntiviralsAntibody medical ingredientsHepatitis c viralCellular antigens
The invention discloses an expression vector PVX-6His-CTBt-Bt for producing a multi-epitope vaccine of hepatitis c virus. The multi-epitope vaccine 6His-CTBt-Bt of hepatitis c virus expressed by the vector comprises a histidine tag, an orally-taken immunologic adjuvant cholera toxin B subunit (CTB) and six B cellular antigen epitopes Bp of refractory hepatitis c virus genetype 1. To facilitate high-efficiency expression of the vaccine in tobacco, the 6His-CTBt-Bt gene optimized by an artificially synthesized codon is cloned to a high-efficiency plant virus expression vector potato X virus vector PVX201 to obtain the recombinant expression vector PVX-6His-CTBt-Bt of the multi-epitope vaccine 6His-CTBt-Bt of the hepatitis c virus; the expression vector is inoculated to the tobacco; the tobacco is regarded as a reactor to produce the anti-hepatitis c virus vaccine which aims to the refractory hepatitis c virus, is easy to purify, and can achieve a good orally-taken immune effect and resist against virus infection in vitro.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Vaccine antigen with increased immunogenicity
ActiveUS10494406B2Low immunogenicityImprove induction efficiencySsRNA viruses positive-senseAntibody mimetics/scaffoldsEscherichia coliVaccine antigen
Owner:IDEMITSU KOSAN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com