Preparation method of cholera toxin B subunit protein having biological activity
A cholera toxin and biological activity technology, applied in the fields of biotechnology and resource utilization and sustainable development, can solve the problems of low expression and unclear biological activity of proteins, and achieve high protein expression, high purity and simple equipment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A preparation method of biologically active cholera toxin B subunit protein, comprising the steps of:
[0047] 1. Construction of a prokaryotic expression vector: the CTB gene (nucleotide sequence shown in SEQ ID NO.1, corresponding amino acid sequence shown in SEQ ID NO.2) is cloned onto the pET28a-OH vector (restriction site BamH I and Xho I), get
[0048] CTB-pET28a-OH expression vector.
[0049] The pET28a-OH vector is obtained by transforming the pET-28a(+) vector. Specifically, the sequence from the N-terminal His-Tag to BamH I of pET-28a(+) is deleted by PCR. This transformation can make the cloned CTB -The N-terminus of the pET28a-OH expression vector only has His-Tag, which can increase the expression of CTB and reduce the restriction enzyme cutting procedure.
[0050] 2. Expression vector transformation: the CTB-pET28a-OH expression vector was transformed into Escherichia coli BL21(DE3).
[0051] 3. Monoclonal activation: Pick a single clone containing the ...
Embodiment 2
[0071] Activity detection of cholera toxin B subunit protein:
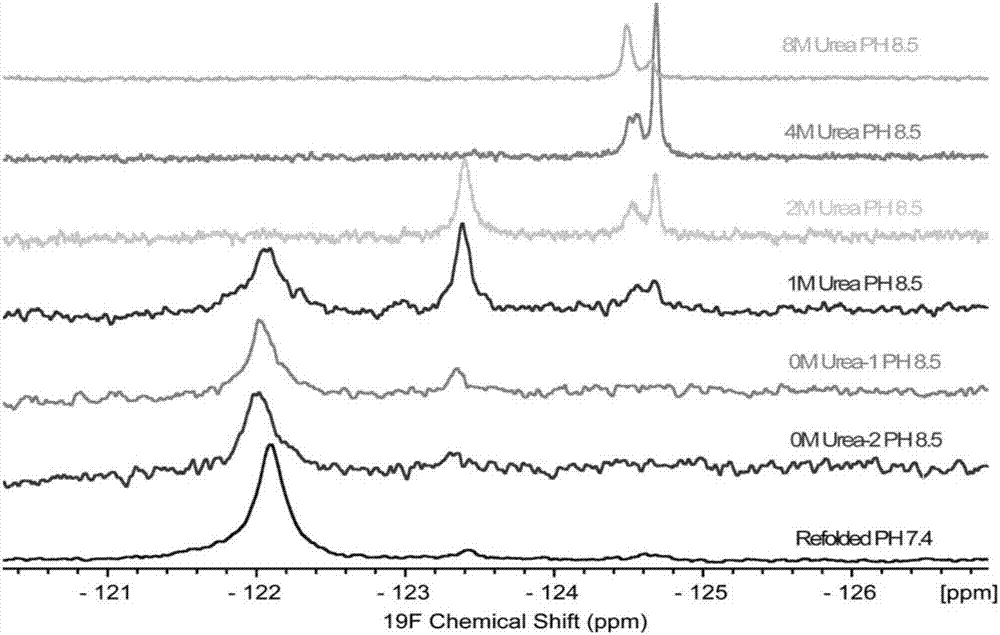
[0072] 1. During the expression of cholera toxin B subunit, in the culture medium, add 5-fluoro-tryptophan to mark the 88th tryptophan of CTB, the purpose is to use 19 F NMR directly observes the renaturation process of inclusion bodies. During the renaturation process, each time the buffer is changed, 450uL of the sample in the dialysis bag is added to 50uL D 2 O, mix well, pack into 5mm NMR tube, adopt fluorine spectrum on 600MHz NMR spectrometer, according to 19 The peak of F can clearly observe the denaturation-intermediate-renaturation process ( figure 1 ). At the same time, take 10uL of each spectrum sample and run SDS-Page, and you can clearly see the appearance of pentamer after CTB renaturation ( figure 2 ).
[0073] 2. The cholera toxin subunit B after refolding, in order to detect its biological activity, we use fluorescein isothiocyanate (FITC) to label the protein to obtain fluorescent FITC-CTB, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com