Design and preparation method and application of novel fasciola hepatica multi-epitope vaccine
A Fasciola hepatica, multi-epitope technology, applied in the field of animal husbandry medicine, can solve problems such as unavoidable repeated treatment, complete deworming of drugs, and increase of drug-resistant worms of Fasciola hepatica
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0050] Example 1: Molecular structure design of Fasciola hepatica multi-epitope vaccine CTB-SAPE
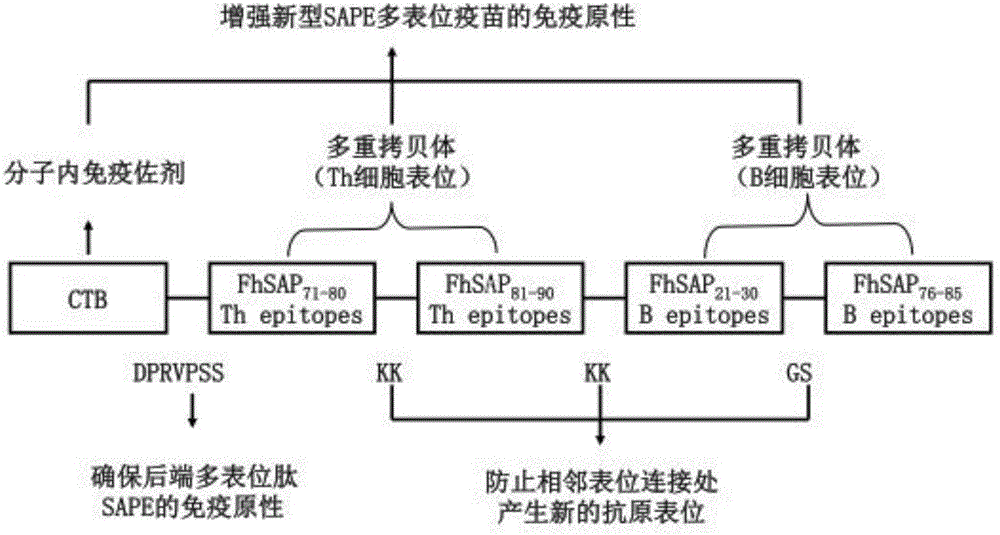
[0051] According to "The body's immune protection mechanism against Fasciola hepatica" and "Immunological properties of saposin-like protein epitopes", select saposin-like protein Th and B cell epitope FhSAP 21-30 、FhSAP 76-85 、FhSAP 71-80 and FhSAP 81-90 Used in the construction of a new type of Fasciola hepatica multi-epitope vaccine. In the design of the new Fasciola hepatica multi-epitope vaccine, our research group placed the Th cell epitope at the front of the B cell epitope, which is helpful for the effective presentation of the B cell epitope, and then through bioinformatics DNAstar and RANKPEP Software analysis and evaluation, the epitope sequence was determined as FhSAP 71-80 -FhSAP 81-90 -FhSAP 21-30 -FhSAP 81-90 ; KK is selected as the spacer sequence of the adjacent Th epitope, and GS is selected as the spacer sequence of the adjacent B epitope; the urease epi...
example 2
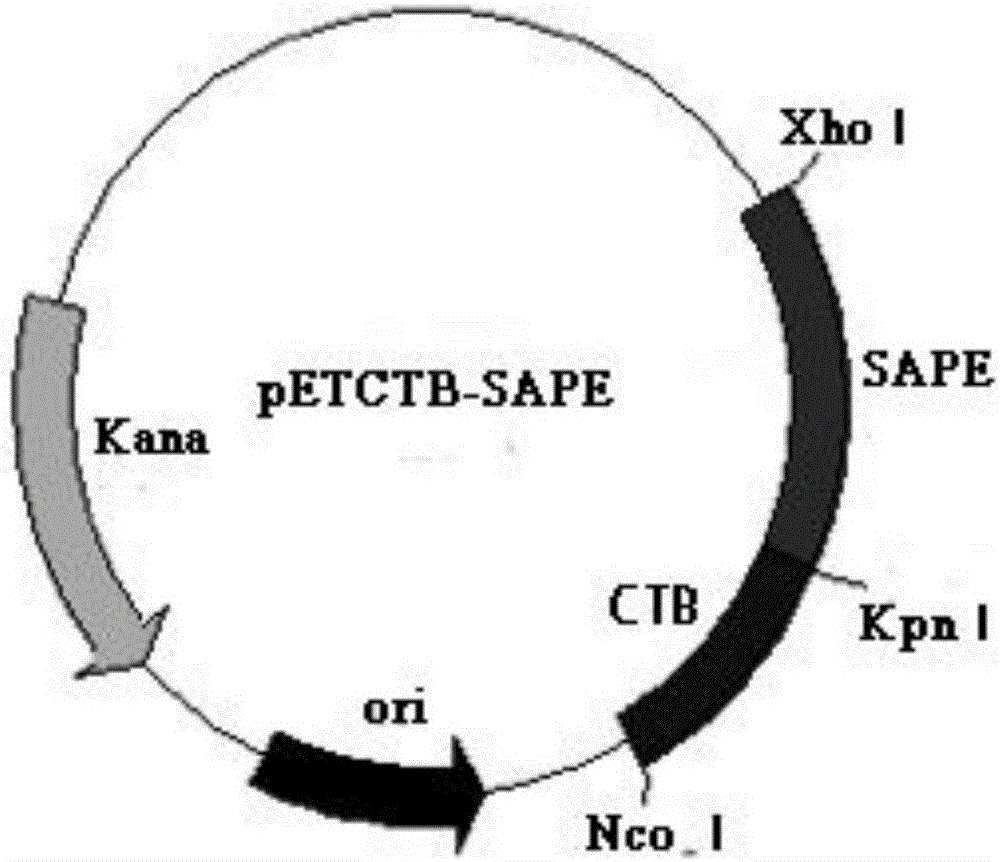
[0053] Example 2: Construction of recombinant expression vector pETCTB-SAPE (containing fusion gene CTB-SAPE)
[0054] (1) Gene synthesis of polyepitope peptide SAPE nucleotide sequence
[0055] The amino acid sequence of the previously designed multi-epitope peptide SAPE was transformed into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and Nanjing GenScript Biotechnology Co., Ltd. was entrusted to carry out gene synthesis.
[0056] (2) Connection of multi-epitope peptide SAPE gene and pETC expression vector
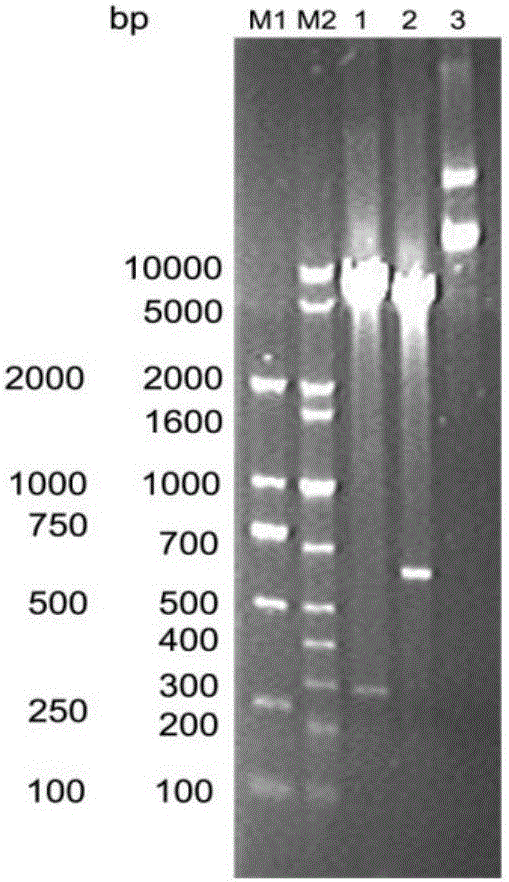
[0057] The recombinant plasmids pUCSAPE and pETCTB (gifted by Dr. Guo Le) were extracted by plasmid mini-extraction kit (Tiangen), and double-digested with Kpn I / Xho I respectively. The double-digestion reaction system is as follows:
[0058] pUCAPE / pETCTB
[0059] After digestion at 37°C for 2 h, electrophoresis was performed on a 1% agarose gel, and the electrophoresis results were observed. The mult...
example 3
[0062] Example 3: Prokaryotic expression of multi-epitope peptide fusion protein CTB-SAPE
[0063]Transform the correct recombinant expression plasmid pETCTB-SAPE into E.coli BL21(DE3) strain. On the pre-prepared LB plate containing 50 μg / mL Kana, inoculate the loop-streaked genetically engineered strain pETCTB-SAPE / BL21(DE3), place it upside down in a 37°C incubator, and after cultivating overnight for 12-16 hours, pick a single colony. Inoculate in LB medium containing 50µg / mL Amp, culture at 37°C, 180rpm, for 12-16h. Inoculate recombinant bacteria with 2% inoculum in LB medium containing 50µg / mL Kana, shake overnight at 37°C and 180rpm, and inoculate the bacterial solution with 1% inoculum in LB liquid containing 50µg / mL Kana the next morning After shaking the flask at 37°C and 180rpm for 3 hours in the culture medium, IPTG was added to make the final concentration reach 1mmol / L, and the expression was induced at 37°C and 180rpm. The empty vector strain pET28a / BL21(DE3) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com