Vaccine for blocking transmission of echinococcosis pathogeny echinococcus granulosus from source
A technology of Echinococcus granulosus and vaccines, applied in the field of vaccines, can solve the problems of accelerating the control and elimination of echinococcosis, achieve the effects of increasing serum titer, enhancing intestinal mucosal immune response, and high immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1, Echinococcus granulosus recombinant CTB-EgM123 vaccine and its immune effect detection
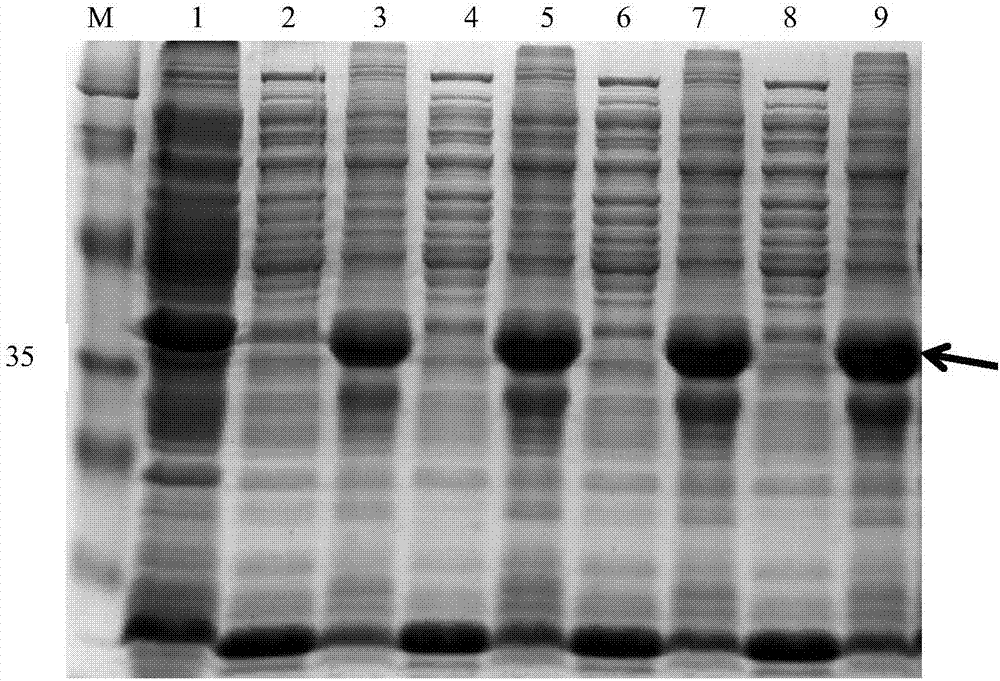
[0048] 1. Preparation of Echinococcus granulosus recombinant CTB-EgM123 vaccine
[0049] 1. Construction of recombinant prokaryotic expression vector
[0050] (1) Construction of recombinant prokaryotic expression vector pET28a / CTB-EgM123
[0051] First, according to the multi-cloning site on the prokaryotic expression vector pET28a, the CTB sequence was introduced into the pET28a vector, specifically: artificially synthesized CTB sequences with restriction sites Nhe I and BamH I at both ends (" GCTAGC +sequence 5+ GGATCC ”), and after digested by Nhe I and BamH I, it was connected to the large backbone fragment of the pET28a vector that had undergone the same digestion, and the intermediate vector pET28a / CTB was obtained after sequencing and verification.
[0052] Then, according to the EgM123 gene coding sequence published on GenBank and specific primers were desig...
Embodiment 2
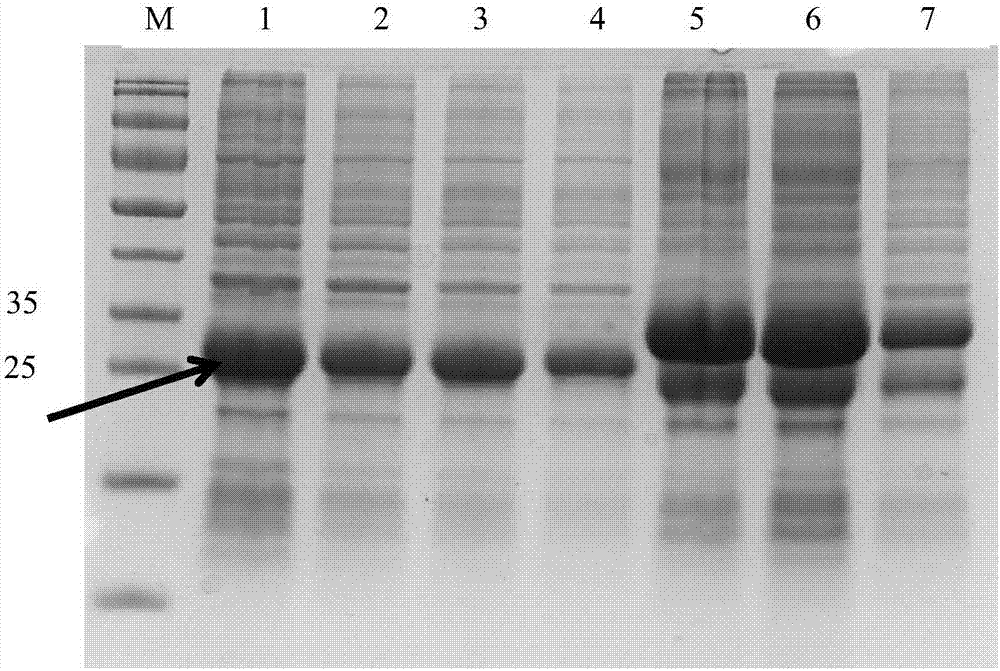
[0091] Example 2. Detection of combined immune effect of Echinococcus granulosus recombinant CTB-EgM123 vaccine and Echinococcus granulosus EgM123 protein recombinant Mycobacterium smegmatis vaccine
[0092] 1. Preparation of Echinococcus granulosus EgM123 protein recombinant Mycobacterium smegmatis vaccine
[0093] 1. Construction of recombinant plasmid pMV261-EgM123
[0094] Primers were designed according to the published EgM123 sequence on GenBank, and the primer sequences were:
[0095] Primer-F2 (the underlined part is the recognition sequence of EcoR I):
[0096] 5'-CAT GAATTC ATGGAACCAGTGAATTTTGCC-3';
[0097] Primer-R2 (the underlined part is the recognition sequence of Sal I):
[0098] 5'-ACT GTC GAC TTATTTCGTCTTTAAGGCACGAAAC-3'.
[0099] Primers were synthesized by Shanghai Bioengineering Technology Service Co., Ltd. The EgM123 gene fragment was amplified using the adult cDNA of Echinococcus granulosus (the total RNA of the adult was extracted and obtained ...
Embodiment 3
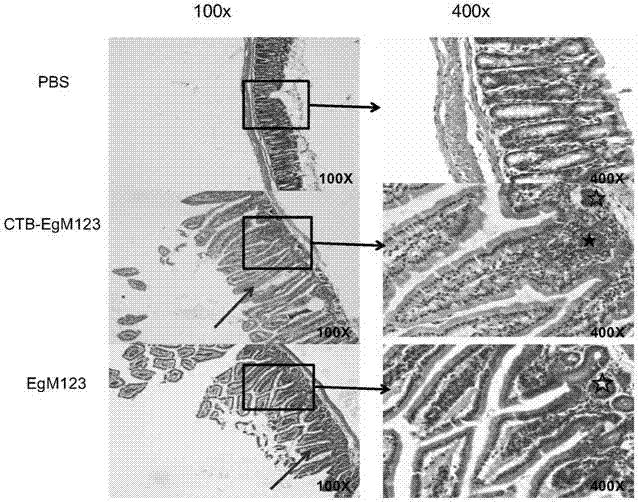
[0112] Example 3, Echinococcus granulosus recombinant CTB-EgM123 vaccine enhances intestinal mucosal immune response
[0113] BALB / c mice were immunized with fusion protein CTB-EgM123 prepared and purified in Step 1 of Example 1, 20 μg / mouse for each immunization, and Freund's adjuvant was added at the same time. Freund's adjuvant (the first time is Freund's complete adjuvant, the second to third time is Freund's incomplete adjuvant) and the fusion protein CTB-EgM123 3 are completely mixed into an emulsion according to the ratio of 1:1 (volume ratio). Subcutaneous immunization. One week after the completion of the three immunizations, the small intestine was washed with normal saline, fixed with 4% paraformaldehyde, and routinely embedded in paraffin, sectioned, and stained with HE using the methods of tissue observation and immunohistochemical detection of the small intestine after immunization. Learn to observe. Goat anti mouse IgA(A90-403P), Goat anti mouse IgG2a(A90-107P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com