Designing and preparing method and application of novel alveolar echinococcosis subunit vaccine
A multilocular echinococcosis and subunit vaccine technology, applied in the field of biomedicine, can solve the problems of few antigen studies, no multilocular hydatid immunotherapy method, complicated immune escape mechanism of multilocular hydatid infection host, etc. , to achieve the effect of enhancing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
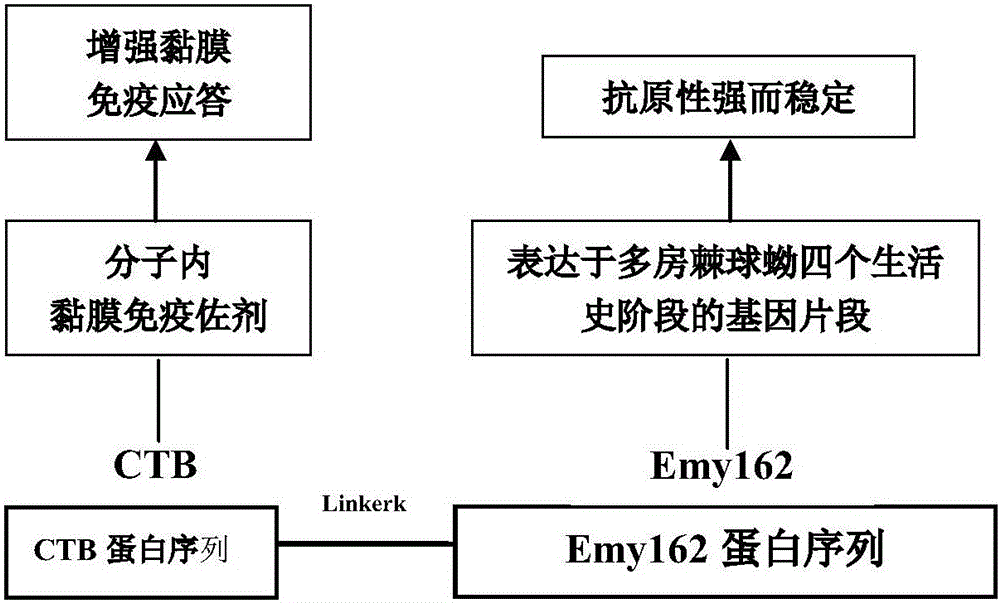
[0045] Example 1: Molecular structure design of multilocular Echinococcus subunit vaccine CTB-Emy162
[0046] On the basis of obtaining the amino acid sequence of Emy162, the intramolecular mucosal immune adjuvant CTB was added on this basis, and a fusion protein of Echinococcus multilocularis with a scientific and reasonable structure was designed to effectively improve the mucosal immune response in vivo.
[0047] Results: The molecular structure design characteristics and ideas of multilocular Echinococcus subunit vaccine CTB-Emy162 are as follows ( figure 1 ) shown.
example 2
[0048] Example 2: Construction of recombinant expression vector (containing fusion gene CTB-Emy162)
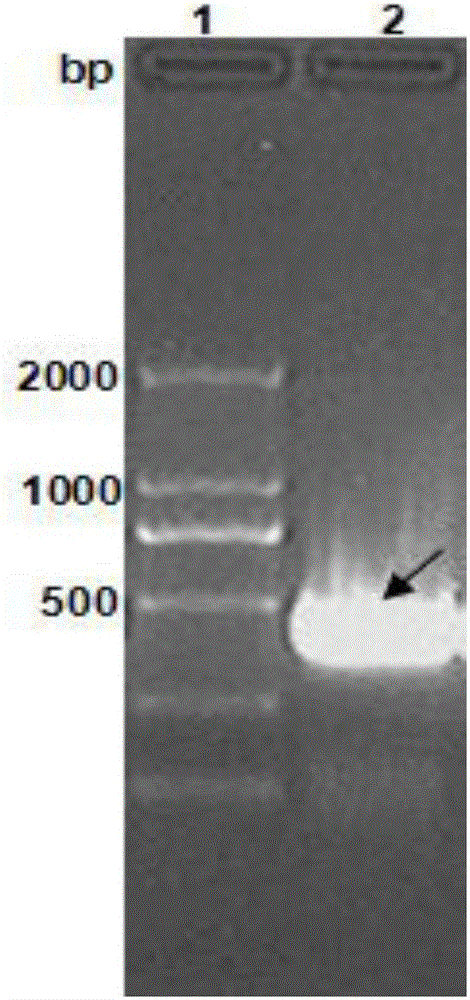
[0049] (1) Gene synthesis of the nucleotide sequence of the surface antigen Emy162 of Echinococcus multilocularis
[0050] The amino acid sequence of Emy162 obtained earlier was transformed into the corresponding nucleotide sequence according to the codon preference principle of Escherichia coli, and Nanjing GenScript Biotechnology Co., Ltd. was entrusted to carry out the gene synthesis. The synthesized Emy162 was subjected to PCR, and the reaction system was as follows:
[0051] Emy162 (0.5mg / mL)
1 µL
Emy162 primer
1 µL
Mix (0.1mg / mL)
10µL
wxya 2 O (No RNase)
8 µL
Total
20 µL
[0052] The PCR reaction conditions were: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30 sec, annealing at 72°C for 30 sec, extension at 72°C for 40 sec, a total of 30 cycles, and a final extension at 72°C for 10 min. Then use...
example 3
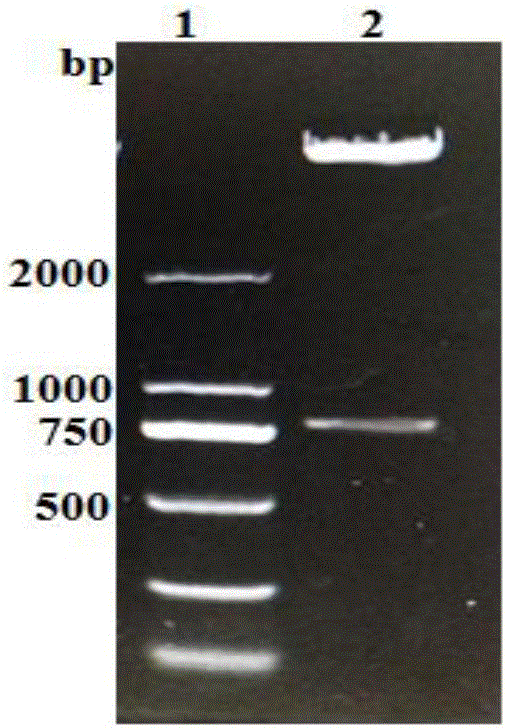
[0062] Example 3 Prokaryotic expression of fusion protein CTB-Emy162 of Echinococcus multilocularis
[0063] The 100% successful recombinant expression plasmid pET28a-CTB-Emy162 was transformed into E.coli BL21(DE3) strain. On the pre-prepared LB plate containing 50ug / mL Kana, inoculate the loop-streaked genetically engineered strain pET28a-CTB-Emy162 / BL21(DE3), place it upside down in a 37°C incubator, and culture it overnight for 12-16 hours, then pick a single Colonies were inoculated in LB liquid medium containing 50µg / mL Kana, cultured at 37°C, 190rpm, for 6-8h. Inoculate recombinant bacteria with 1% inoculum in LB liquid medium containing 50 µg / mL Kana, shake overnight at 37°C and 180 rpm, and inoculate the bacterial solution at 1% inoculum in LB containing 50 µg / mL Kana the next morning In liquid medium, after shaking the flask at 37°C and 190rpm for 9h, add IPTG to make the final concentration reach 1mmol / L, induce expression at 28°C and 190rpm, and use the empty vect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com