Immunomodulation agent with adjuvant having function for treating atherosclerosis
A technology for atherosclerosis and immunomodulators, applied in the fields of expression, separation and purification, immunomodulators, and fusion protein CTB-p277, which can solve the problems of weak immunogenicity of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
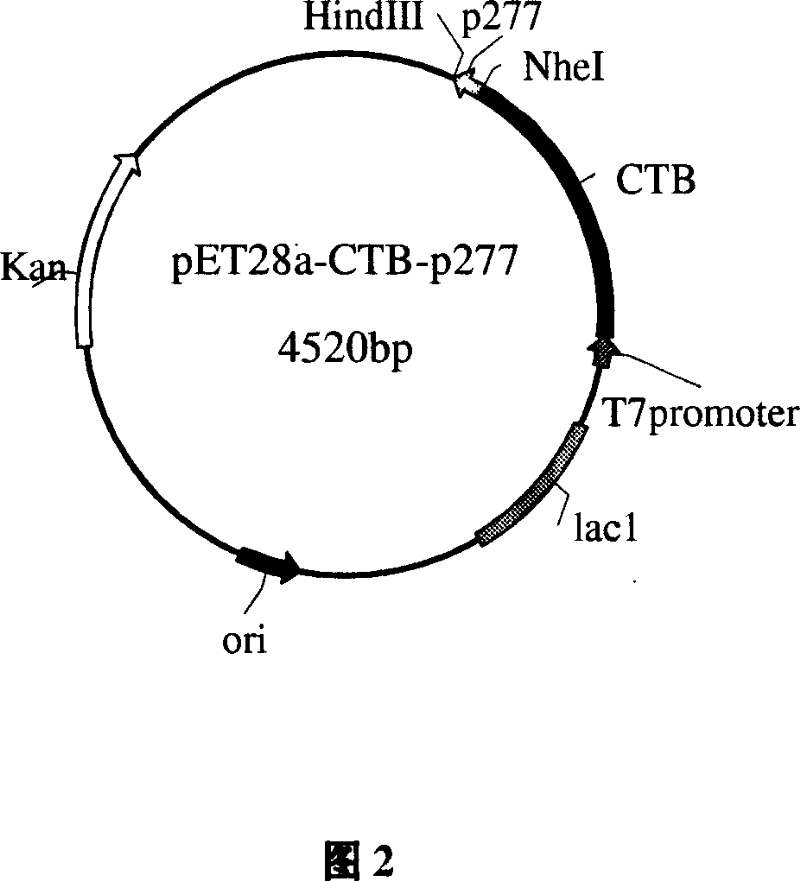
[0065] Embodiment 1: Design, synthesis and cloning of CTB-p277 polypeptide gene
[0066] 1. Acquisition of CTB gene
[0067] On the premise of not changing the CTB gene, using the pET28a-CTB plasmid as a template, the upstream primer P1 and the downstream primer P2 were designed with computer aids. Synthesized by Shanghai Boya Biotechnology Co., Ltd. The pET28a-CTB plasmid was donated by the Department of Microbiology, China Pharmaceutical University.
[0068] Upstream primer P1: 5'GGG TCATGA CACCTCAAAAATATTACTG 3'
[0069] PagI
[0070] Downstream primer P2: 5'AAA GCTAGC ATTTGCCATACTAATTGCGGCAATCGC 3'
[0071] NheI
[0072] A PagI restriction site was introduced into the upstream primer P1, and a NheI restriction site was introduced into the downstream primer P2.
[0073] The pET28a-CTB plasmid was used as a template, and P1 and P2 were respectively used as upstream and downstream primers for PCR amplification.
[0074] The PCR re...
Embodiment 2
[0078] Embodiment 2: Expression of CTB-p277 gene in Escherichia coli
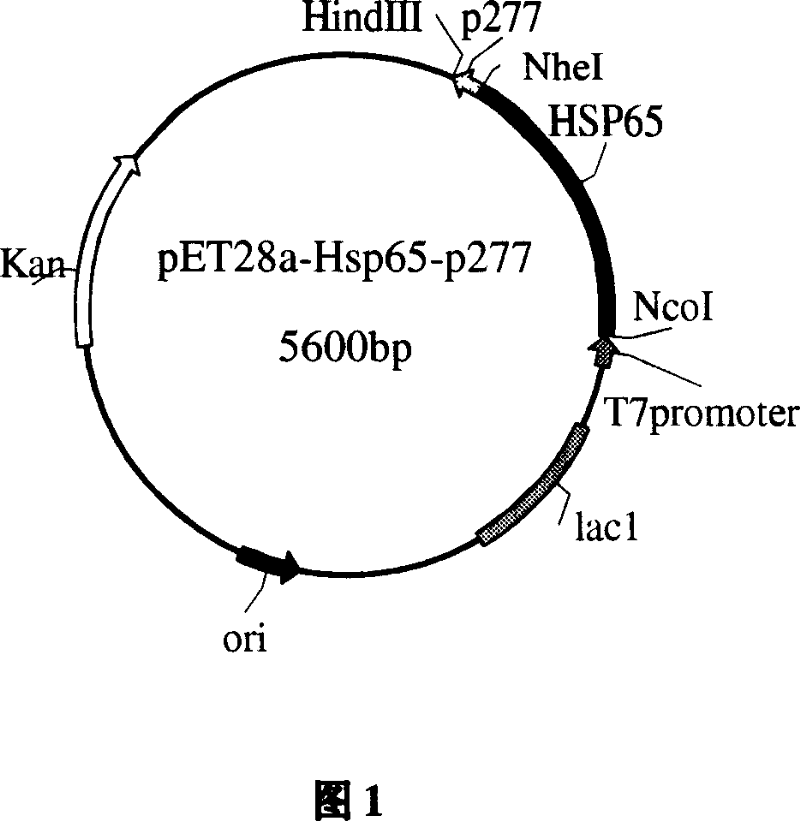
[0079] The recombinant plasmid pET28a-CTB-p277 was transformed into Escherichia coli BL21. Pick single bacterium colony and inoculate containing 50ug / ml kanamycin LB liquid culture medium from growing different transformant plates, cultivate overnight at 37 DEG C of constant temperature shaking, transfer and inoculate into fresh corn steep liquor liquid culture medium by 1% ratio ( 50ug / ml kanamycin), after culturing at 37°C for 4 hours, adding α-lactose at a final concentration of 0.5mmol / L to induce the expression of T7 RNA polymerase in Escherichia coli, and continuing to culture to express the fusion protein CTB-p277. A small amount of bacterial liquid was taken 6 hours after induction, and the bacterial cells were recovered by centrifugation. SDS-PAGE electrophoresis and thin-layer scanning showed that the fusion expression of CTB-p277 polypeptide gene had been realized. The fusion protein accounts fo...
Embodiment 3
[0080] Example 3: Separation and purification of recombinant protein CTB-p277
[0081] The engineered bacteria after induced expression were recovered by centrifugation, suspended in 50ml of 50mmol / L Tris-HCl buffer per 10g of wet bacteria, added 80μl lysozyme (10mg / ml) to each gram of wet bacteria, and then added Triton X- 100 to a final concentration of 0.5%, and shake overnight at 37°C. Add 0.5 μl DNase I (1 mg / ml) to each gram of wet bacteria, and continue shaking at 37°C for 45 minutes. Centrifuge at 10000r / min for 15min, the target protein forms inclusion bodies and appears in the precipitate, discard the supernatant.
[0082] According to the wet weight of each gram of inclusion body, add 10ml of washing solution I to resuspend the precipitate evenly, discard the supernatant after centrifugation, add washing solution II to the precipitate and wash in the same way, and finally wash off the residual washing solution II with distilled water, and precipitate For standby, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com