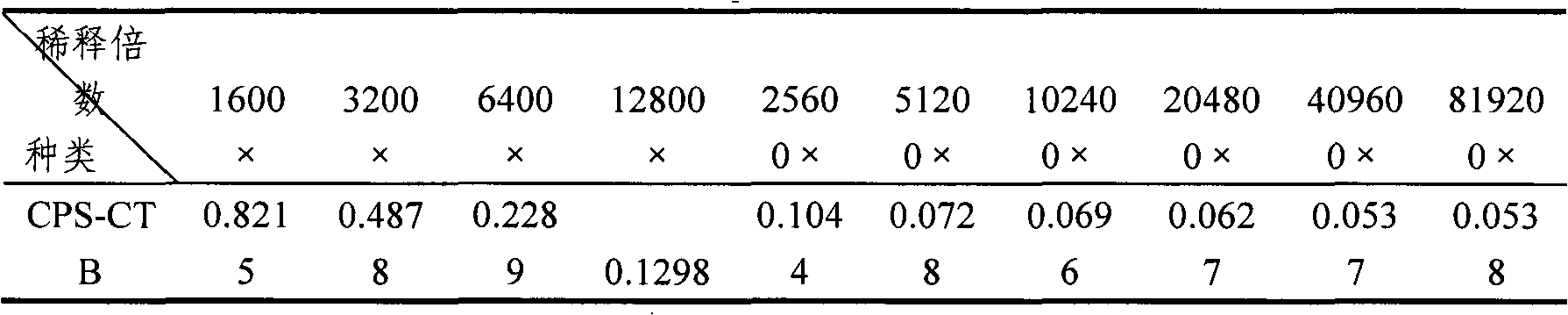Vibrio cholerae O139 capsular polysaccharide conjugate vaccine and preparation method thereof
A Vibrio cholerae-conjugated vaccine technology, applied in the direction of carrier-bound antigen/hapten components, pharmaceutical formulations, antibacterial drugs, etc., can solve the problems of expensive treatment, inability to control the occurrence and spread of diseases, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
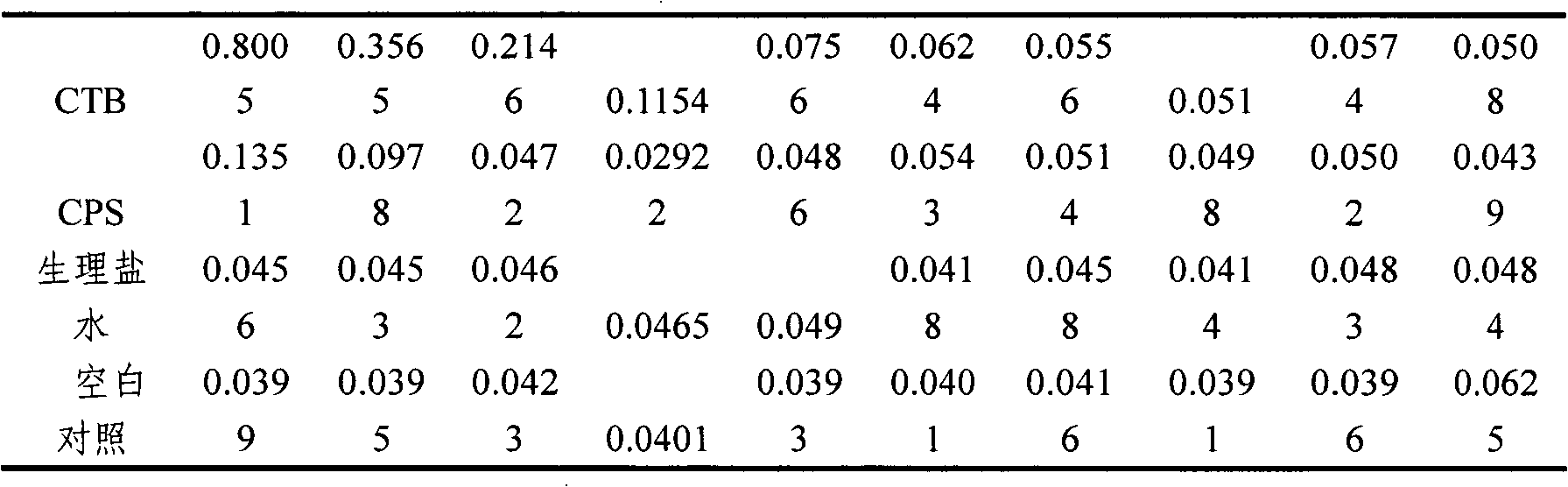
[0024] Example 1 Preparation of Vibrio cholerae O139 capsular polysaccharide
[0025] Take Vibrio cholerae O139 (V.cholerae, CMCC 93-3) (purchased from the Chinese Medical Bacteria Preservation and Management Center) freeze-dried working seed batch strain, suspend it in ordinary meat water, shake culture at 37°C for 5h, and take it with an inoculation loop Activated liquid strains, streaked and inoculated on solid plates to separate individual colonies, cultured at 37°C for 16-18h, randomly picked colonies for slide agglutination test; picked well-agglutinated colonies, cultured in a small amount of liquid, and then transferred Into the small fermentation tank to prepare the seed liquid, and then aseptically inject it into the large culture tank, where the fermentation medium adopts trypsin digestion-thick Jingle medium, the inoculum is 5%-10%, and the culture temperature is 30 -35℃, aeration and stirring, the pH value is maintained at about 7.6-8.0. The seed pot is cultivated f...
Embodiment 2
[0027] Example 2 Construction of Vibrio cholerae B subunit engineering bacteria
[0028] 1. Design primers according to the B subunit coding gene of Vibrio cholerae published by NCBI (GenBank accession number: EU854477.1), and the upstream primer CTB-F: GATAT ATGATTAAATTAAAATTTG (SEQ ID No.1), downstream primer CTB-R: CCG TTAATTTGCCATACTAATTGC (SEQ ID No. 2);
[0029] 2. Using the genomic DNA of Vibrio cholerae O1 strain V.cholerae CMCC 569B (purchased from the Chinese Medical Bacteria Collection and Management Center) as a template, a 375bp coding gene fragment of Vibrio cholerae B subunit was obtained by PCR amplification;
[0030] 3. Double-enzyme digestion of the target gene fragment with Bgl II and Xho I, ligate it with the expression vector pET-22b digested with the same endonuclease, and introduce it into the host cell E.coli BL21(DE3), and get it after screening The engineered strain that efficiently secretes and expresses the B subunit of cholera toxin, named: E.coli MHCTB...
Embodiment 3
[0032] Example 3 Preparation of Vibrio cholerae B subunit
[0033] The preparation of cholera B subunit is divided into several stages: seed liquid preparation, large tank culture, centrifugation, concentration and purification, filtration sterilization and cryopreservation.
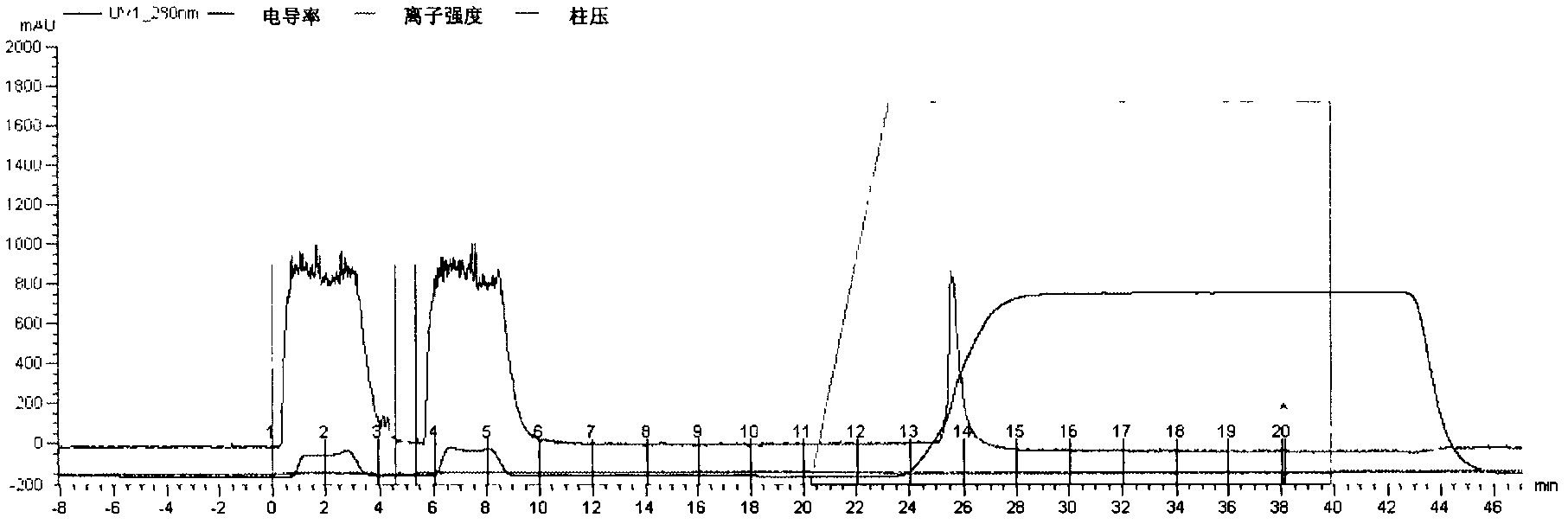
[0034] First, inoculate the working seed batch strain prepared in Example 2 in fresh LB liquid medium containing 50μg / mL ampicillin at a final concentration, and cultivate it overnight at 32°C to prepare seed liquid for inoculation in the fermentor. Fermentation can select suitable large intestine Bacillus growth medium. In the fermenter, when the bacteria grow to OD 600 =20, add IPTG at a final concentration of 0.2mmol / L to induce, continue to culture for 6-8h, stop the culture, adjust the pH of the fermentation broth to 6.5, 8000rpm, 4℃ continuous centrifugation, collect the supernatant, not more than 10kD ultra The CTB solution was concentrated by the filter membrane and purified by ion exchange chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com