Composition for caspase inhibitor prodrug injection
A composition and injection technology, applied in the directions of drug combination, drug delivery, antipyretic drugs, etc., can solve the problems of in vitro drug release period limitation, loss of drugs, low encapsulation efficiency, etc., and achieve improved encapsulation efficiency and release period. added effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
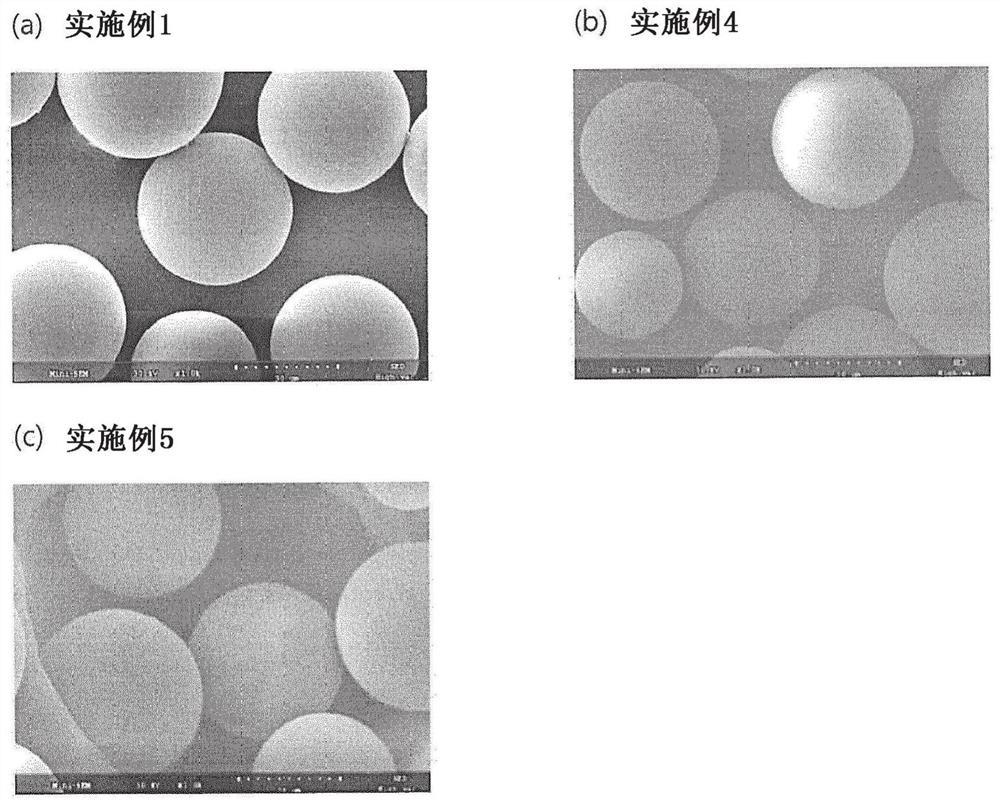
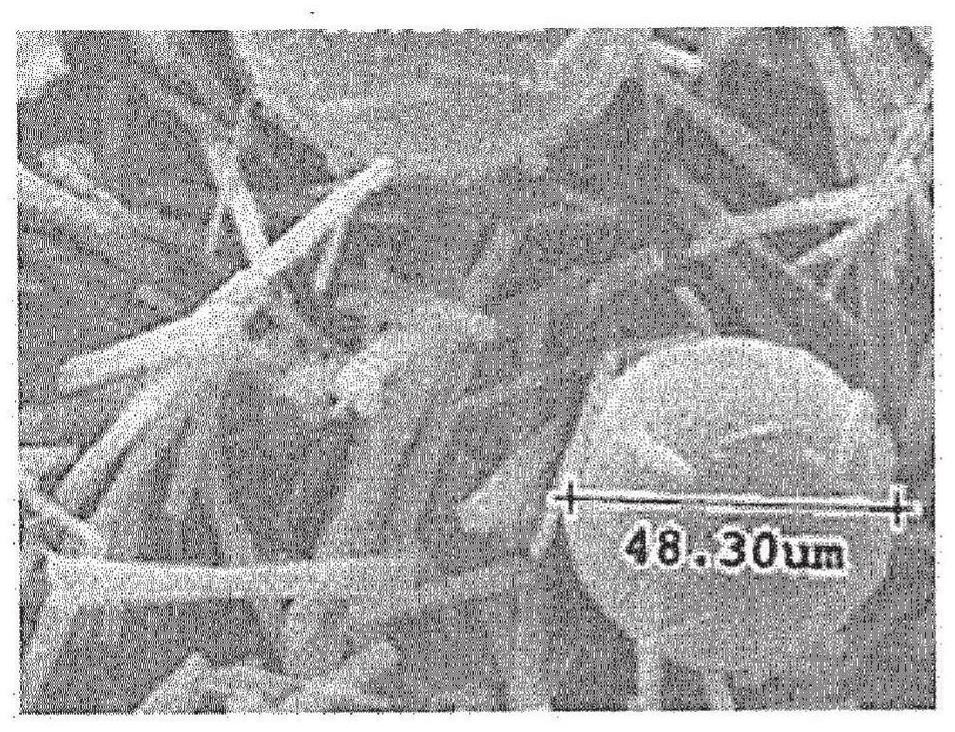
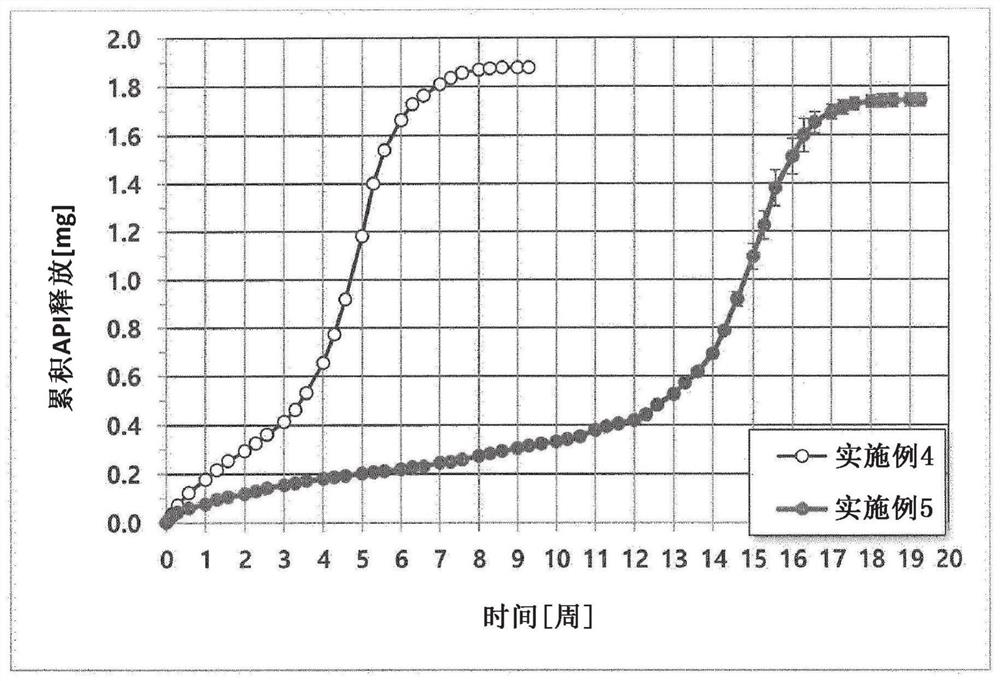
Image
Examples
preparation example 1
[0058] Preparation 1: (2S,3S)-2-(fluoromethyl)-3-((R)-5-isopropyl-3-(isoquinolin-1-yl)-4,5-diacetic acid hydrogen iso Azole-5-carboxamido)-5-oxotetrahydrofuran-2-yl ester
[0059]
[0060] Niflecagen ((R)-N-((2S,3S)-2-(fluoromethyl)-2-hydroxy-5-oxotetrahydrofuran-3-yl]-5-isopropyl-3- (isoquinolin-1-yl)-4,5-dihydroiso Azole-5-carboxamide; 5.0g, 12.0mmol) was dissolved in dichloromethane (50mL), and then acetyl chloride (0.94mL, 13.2mmol, 1.1 equivalents), triethylamine (2.52mL, 18.0mmol, 1.5 eq) and 4-dimethylaminopyridine (0.15 g, 1.2 mmol, 0.1 eq) while maintaining the temperature at 5°C or lower. The reaction mixture was stirred at 25 °C for about 2 hours and quenched by the addition of 10% aqueous sodium bicarbonate (25 mL). After adding water (25 mL) and stirring, the organic layer was separated and distilled under reduced pressure. The resulting mixture was recrystallized in a 1:5 mixture of ethyl acetate and hexane (EtOAc:hexane=1:5) to obtain 3.0 g (yield...
preparation example 2
[0062] Preparation 2: Propionic acid (2S,3S)-2-(fluoromethyl)-3-((R)-5-isopropyl-3-(isoquinolin-1-yl)-4,5-di hydrogen iso Azole-5-carboxamido)-5-oxotetrahydrofuran-2-yl ester
[0063]
[0064] Niflecason (1.0g, 2.4mmol) was dissolved in dichloromethane (20mL), then propionyl chloride (0.23mL, 2.65mmol, 1.1 equivalents), triethylamine (0.5mL, 3.61mmol, 1.5 eq) and 4-dimethylaminopyridine (0.03g, 0.24mmol, 0.1 eq) while maintaining the temperature at 5°C or lower. The reaction mixture was stirred at 25°C for about 2 hours and quenched by the addition of 10% aqueous sodium bicarbonate (10 mL). After adding water (10 mL) and stirring, the organic layer was separated and distilled under reduced pressure. The obtained mixture was subjected to column separation by using a 1:2 mixture of ethyl acetate and hexane (EtOAc:hexane=1:2), to obtain 0.25 g (yield: 23%) of the title compound.
[0065] 1 H NMR (400 MHz, CDCl 3 )δ9.12(d,1H),8.55(d,1H),7.87(d,1H),7.74-7.69(m,3H),7....
preparation example 3
[0066] Preparation 3: Isobutyric acid (2R,3S)-2-(fluoromethyl)-3-((R)-5-isopropyl-3-(isoquinolin-1-yl)-4,5- dihydroiso Azole-5-carboxamido)-5-oxotetrahydrofuran-2-yl ester
[0067]
[0068] Niflecason (1.0g, 2.4mmol) was dissolved in dichloromethane (20mL), and then wasobutyryl chloride (0.73g, 2.65mmol, 1.1 equivalents), triethylamine (0.5mL, 3.61mmol, 1.5 eq) and 4-dimethylaminopyridine (0.03 g, 0.24 mmol, 0.1 eq) while maintaining the temperature at 5°C or lower. The reaction mixture was stirred at 25°C for about 2 hours and quenched by the addition of 10% aqueous sodium bicarbonate (10 mL). After adding water (10 mL) and stirring, the organic layer was separated and distilled under reduced pressure. The obtained mixture was subjected to column separation by using a 1:2 mixture of ethyl acetate and hexane (EtOAc:hexane=1:2), to obtain 0.06 g (yield: 5%) of the title compound.
[0069] 1 H NMR (400MHz, CDCl 3 )δ9.12(d,1H),8.55(d,1H),7.88(d,1H),7.74-7.69(m,3H),...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap