Htra1 modulation for treatment of AMD
A technology of regulatory regions and target sequences, applied in the fields of biology and medical applications, can solve problems such as no therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
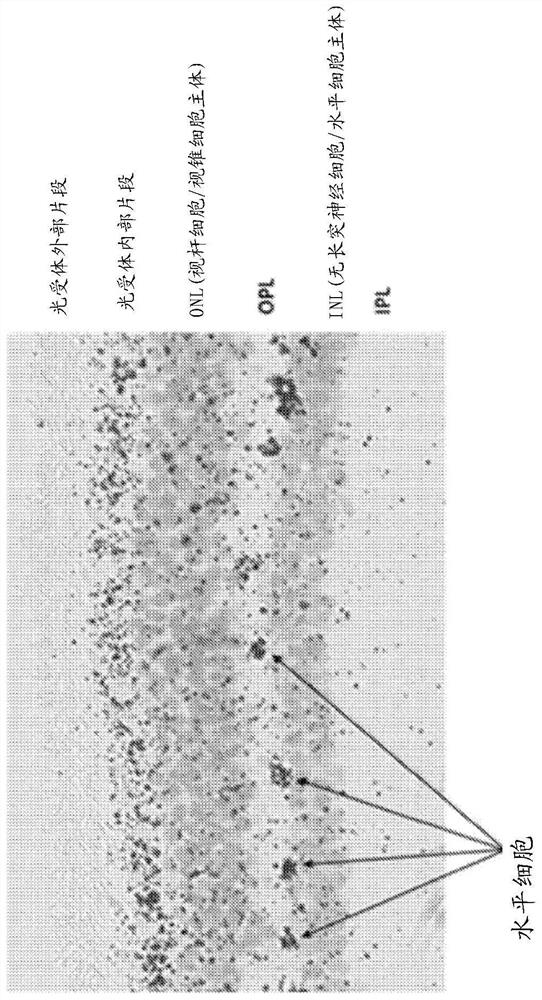
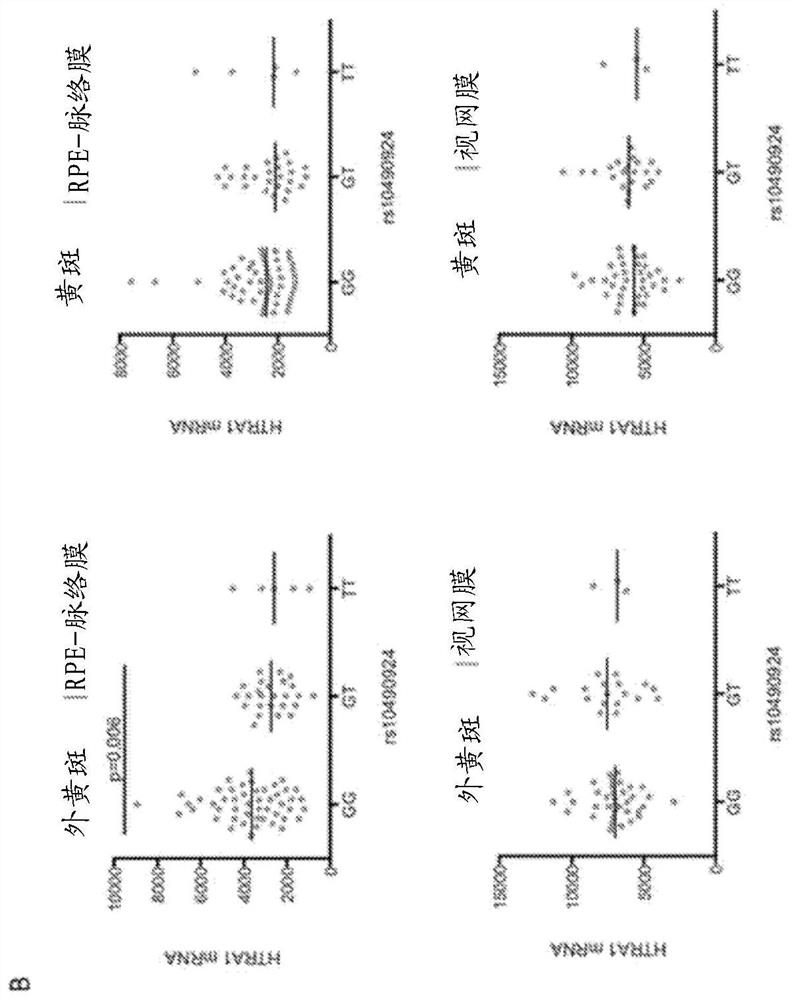
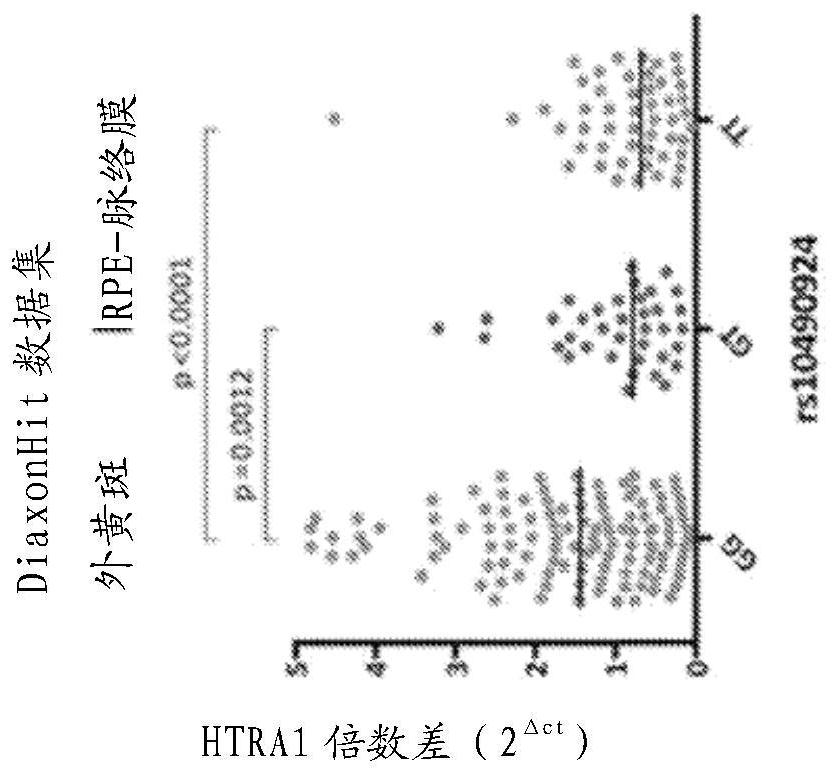
[0064] The inventors have discovered that increasing HTRA1 mRNA and / or protein levels in the eye (eg, retinal pigment epithelium) provides a beneficial effect in subjects suffering from or at risk of developing age-related macular degeneration (AMD). In particular, increasing HTRA1 mRNA and / or protein levels provides a beneficial effect in patients suffering from the manifestations of chromosome 10-directed AMD (or "Chr 10AMD") or who are genetically predisposed to develop chromosome 10-directed AMD.
[0065] The present disclosure provides methods and agents for treating age-related macular degeneration (AMD), preventing its development, slowing its progression, reversing or ameliorating its symptoms and signs by increasing the expression or amount of HTRA1 in the eyes of a subject in need of treatment .
[0066] Methods of treating Chr 10AMD, preventing its development, slowing its progression, reversing or ameliorating its signs and symptoms are described. The method compr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com