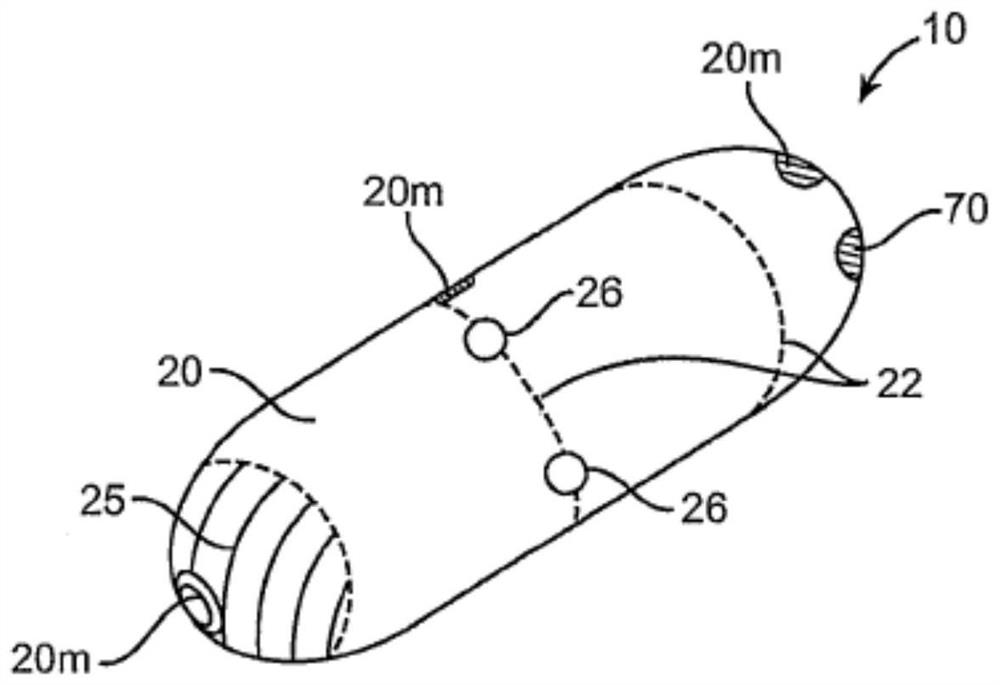
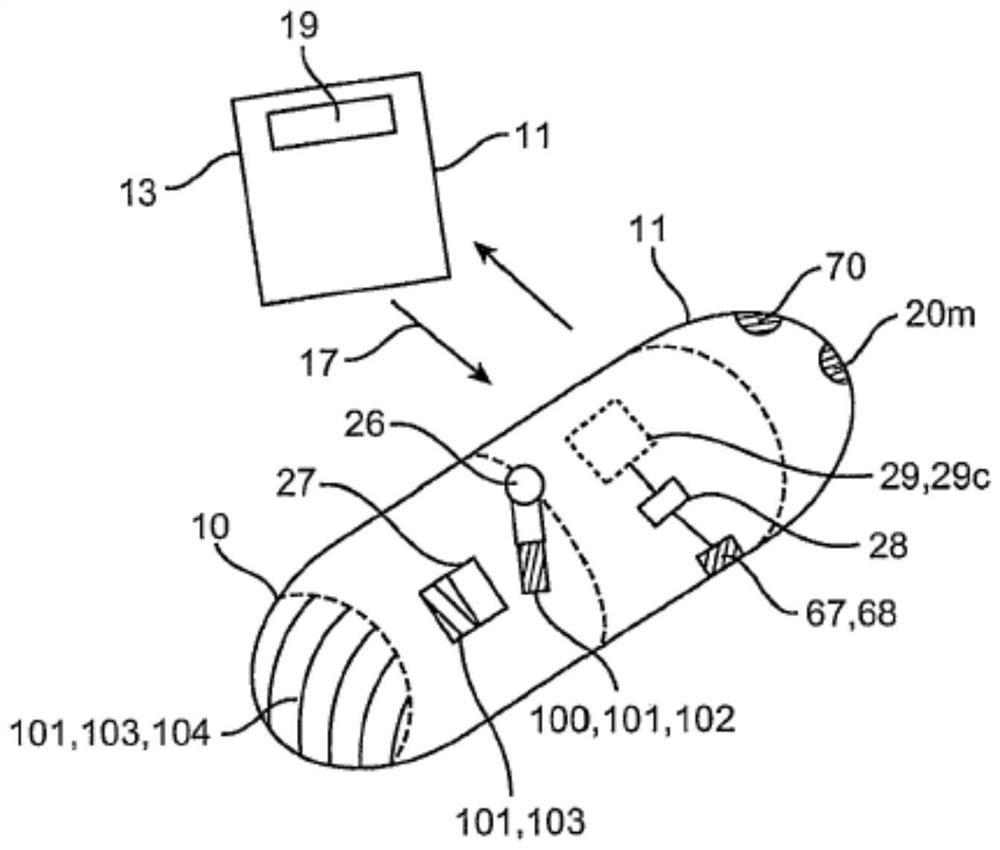
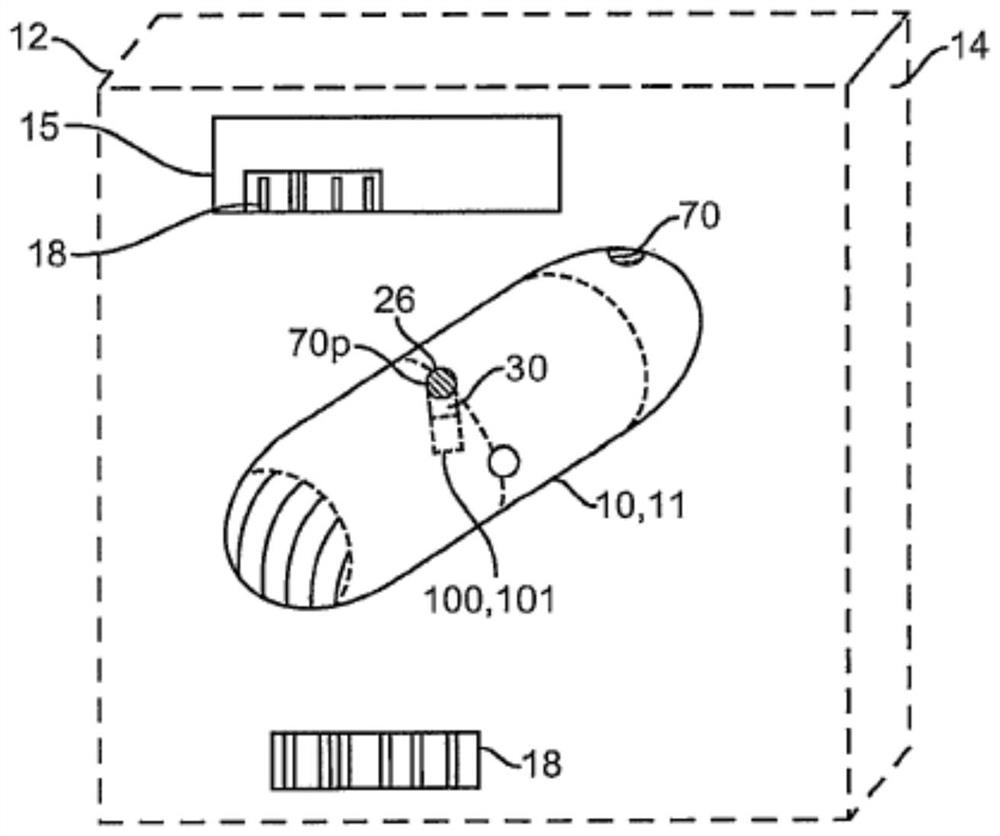
Clotting factor preparations for delivery into tissue of the intestinal tract using a swallowable drug delivery device
A preparation and factor technology, applied in the field of pharmaceutical preparations that can be delivered orally in a stable form of factor VIII, can solve the problems of increased immunogenicity and increased production of inhibitory antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0264] In a specific embodiment, the invention provides therapeutic formulations for the treatment of coagulation disorders comprising a stabilized form of Factor VIII (or other coagulation factors) to increase the drug's circulating half-life and thereby increase its AUC and bioavailability. Stabilization can be achieved by one or both of the following methods: i) chemically complexing a given type of Factor VIII (e.g., wild-type or recombinant) with a stabilizer, or ii) in a therapeutic formulation comprising Factor VIII 100 comprising an excipient comprising a stabilizer. Examples of chemical complexation methods and reagents include pegylation, where FVIII molecules are complexed with PEG molecules, or via Fc fusion, both of which are described in more detail herein. Other stabilizers for chemical complexation are also contemplated, including, for example, von Willebrand factor or one or more hydrogels.
[0265]In various embodiments, stabilizers / excipients may be heterog...
Embodiment 1
[0391] Example 1: In vivo modeling of Factor VIII delivery in humans using an embodiment of the swallowable device described herein
[0392] A pharmacokinetic model was developed to determine human plasma concentration versus time profiles (also described as plasma-time curve or plasma concentration curve) and various pharmacokinetic parameters, including but not limited to T max , T1 / 2, area under the curve (AUC) and absolute bioavailability (F, expressed as a percentage). The model assumes a delivered dose of 100 IU / kg body weight for each drug. Numerical results for PEGylated FVIII (eg, ADYNOVATE) and specifically for ESPEROCT are shown in Tables 6 and 7, respectively. With the exception of bioavailability, the range for each parameter is assumed to be ±25% of the nominal value, with a narrower range of ±10%. The plasma concentration-time profiles of PEGylated FVIII and ESPEROCT are specifically shown in Figures 22 and 23, respectively. The ±25% range shown for each cur...
Embodiment 2
[0399] Example 2: In vivo canine study of PEGylated FVIII delivery
[0400] Purpose: The purpose of the study was to demonstrate peritoneal delivery of the Factor VIII molecule in conscious hemophiliac dogs and to assess the bioavailability of delivery in the peritoneum as Factor VIII via the embodiments and / or variants of the swallowable capsules described herein. Proof of concept study of delivery to the peritoneal cavity. PEGylated FVIII was used as a representative of the Factor VIII molecular class.
[0401] Materials and methods
[0402] Dogs with hemophilia A (Irish Setters) were injected intravenously (IV) and intraperitoneally (IP) with pegylated FVIII. The study was divided into three parts (Part 1, Part 2, Part 3) that varied by dose, time of delivery (days) and type of delivery (IP vs IV). For this example, doses are expressed in IU / kg, it being understood that kg refers to kg of body weight.
[0403] In part 1, dogs received 300 IU / kg by IP route on day 0 (IP ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap