A kind of preparation method of lithium trialkyl borohydride
A technology of trialkyl boron and trimethoxy aluminum hydride, which is applied in the field of preparation of trialkyl lithium borohydride, can solve the problems of long production cycle, difficult solid-liquid separation, and many impurities in tetrahydrofuran solution, and achieve crystal grain The effects of long crystallization time, high purity of solution products, and difficulty in solid-liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention discloses a preparation method of lithium trialkylborohydride, which can be realized by those skilled in the art by referring to the content of the present invention, combining relevant principles of organic chemistry, and appropriately improving process parameters. It should be particularly pointed out that all similar substitutions and modifications apparent to those skilled in the art are deemed to be included within the scope of the present invention. The application of the present invention has been described through the preferred embodiments, and it is obvious that relevant persons can make changes or appropriate changes and combinations of the methods and applications described herein without departing from the content, spirit and scope of the present invention, so as to realize and apply the present invention. Invention technology.
[0024] For a better understanding of the invention and not to limit the scope of the invention, all numbers ...
Embodiment 1
[0027] Example 1: Preparation of lithium triethylborohydride
[0028] Step A: Preparation of the reaction solution: In the ingredient A tank, 115.2 g (0.9 mol) of lithium trimethoxyaluminum hydride was dissolved in tetrahydrofuran ether solution to form 1.3 L of a 0.7 mol / L lithium trimethoxy aluminum hydride solution; in the ingredient B tank, 88.2g (0.9mol) of triethylborane was dissolved in tetrahydrofuran to form 1L of 0.9mol / L triethylborane solution;
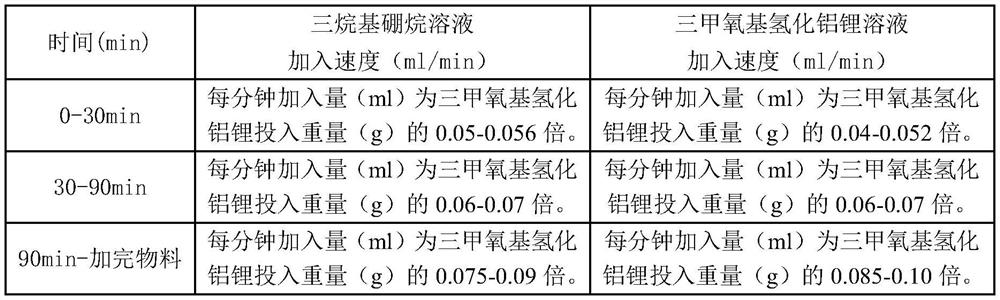
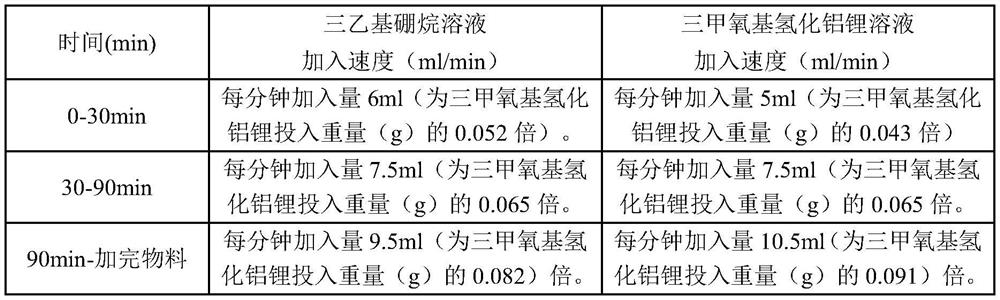
[0029] Step B: Condensation reaction: add 0.3 L (23%) of the lithium trimethoxyaluminum hydride solution prepared in step A into the reaction kettle, stir and control the temperature to 21-23°C, then, follow the steps in Table 1. Dropping scheme, the remaining solution in batching tank A and the solution in batching tank B are added dropwise to the reaction kettle respectively, the whole dropping process is about 130min, after the dropwise addition is completed, the reaction is stirred for 0.5h, and then allowed to stand f...
Embodiment 2
[0032] Example 2: Preparation of Lithium Triisobutyl Borohydride
[0033] Step A: Preparation of the reaction solution: In the ingredient A tank, 115.2 g (0.9 mol) of lithium trimethoxyaluminum hydride was dissolved in tetrahydrofuran ether solution to form 1.3 L of a 0.7 mol / L lithium trimethoxy aluminum hydride solution; in the ingredient B tank, 163.8g (0.90mol) of triisobutylborane was dissolved in tetrahydrofuran to form 1L of 0.9mol / L triisobutylborane solution;
[0034] Step B: Condensation reaction: add 0.26 L (20%) of the trimethoxyaluminum hydride lithium hydride solution prepared in step A into the reaction kettle, stir and control the temperature to 21-23° C. Add scheme, the remaining solution in batching tank A and the solution in batching tank B are added dropwise to the reaction kettle respectively, and the whole dropwise addition process is about 125min. Separation to obtain a supernatant, which is a solution of triisobutyl lithium borohydride in tetrahydrofur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com