Use of FGFR inhibitors in FGFR genetically altered cancers to enhance patient response to immune checkpoint inhibitors in sequential treatment settings
An immune checkpoint, FGFR2 technology, in the field of using FGFR inhibitors in cancers with FGFR genetic alterations to enhance patient response to immune checkpoint inhibitors in a sequential therapy setting, addressing suboptimal clinical outcomes, mUC unmet treatment needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0097] Example 1. Clinical Research
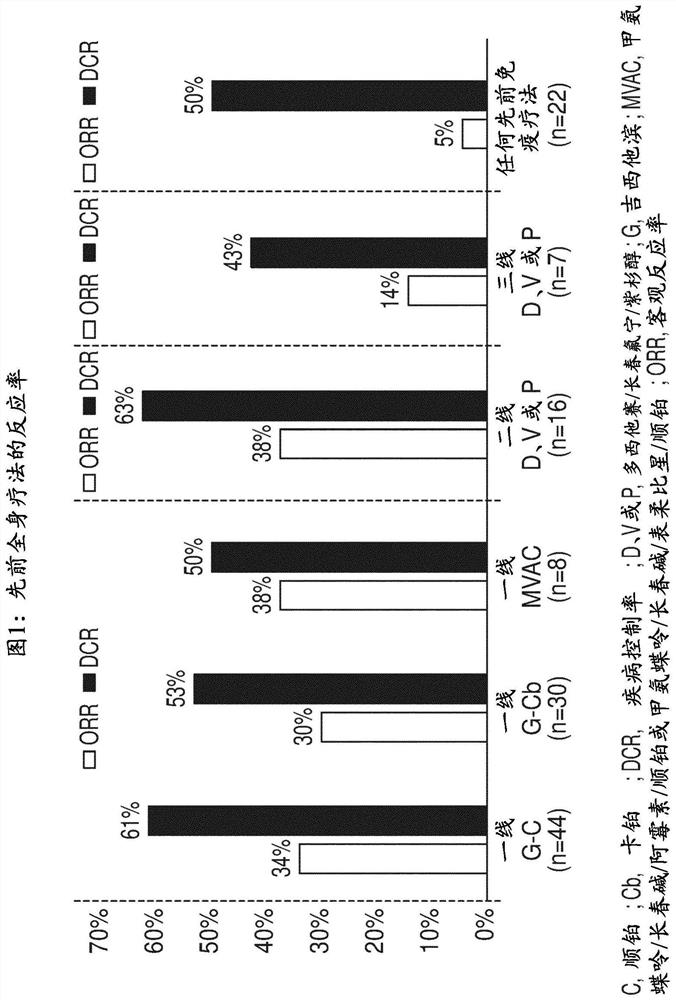
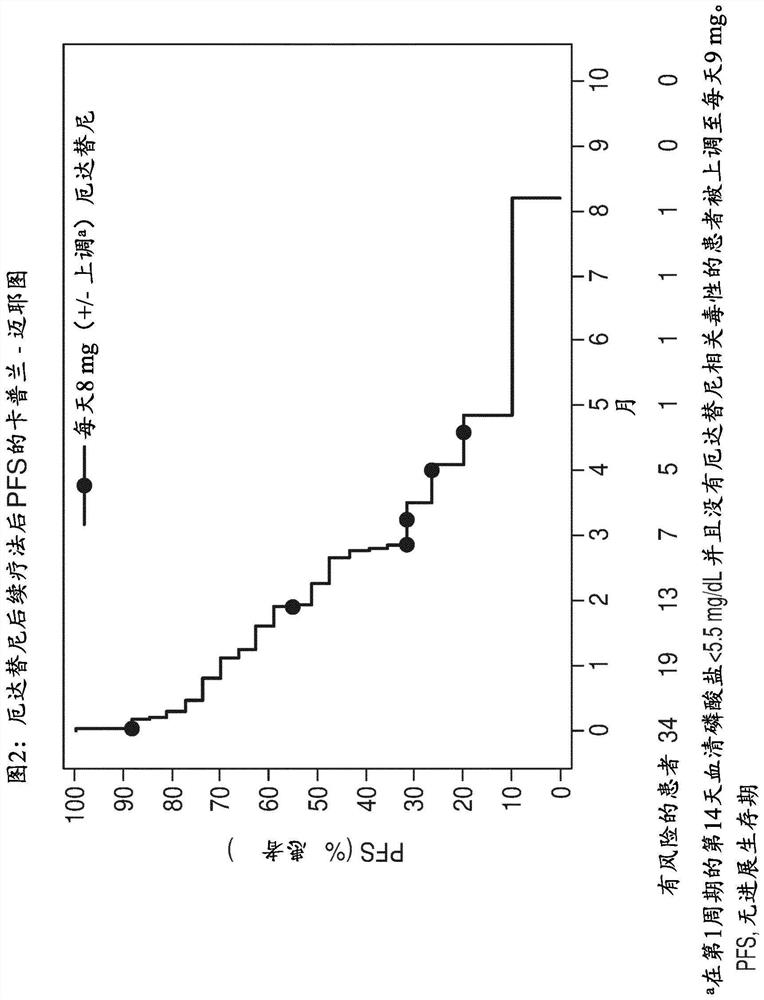
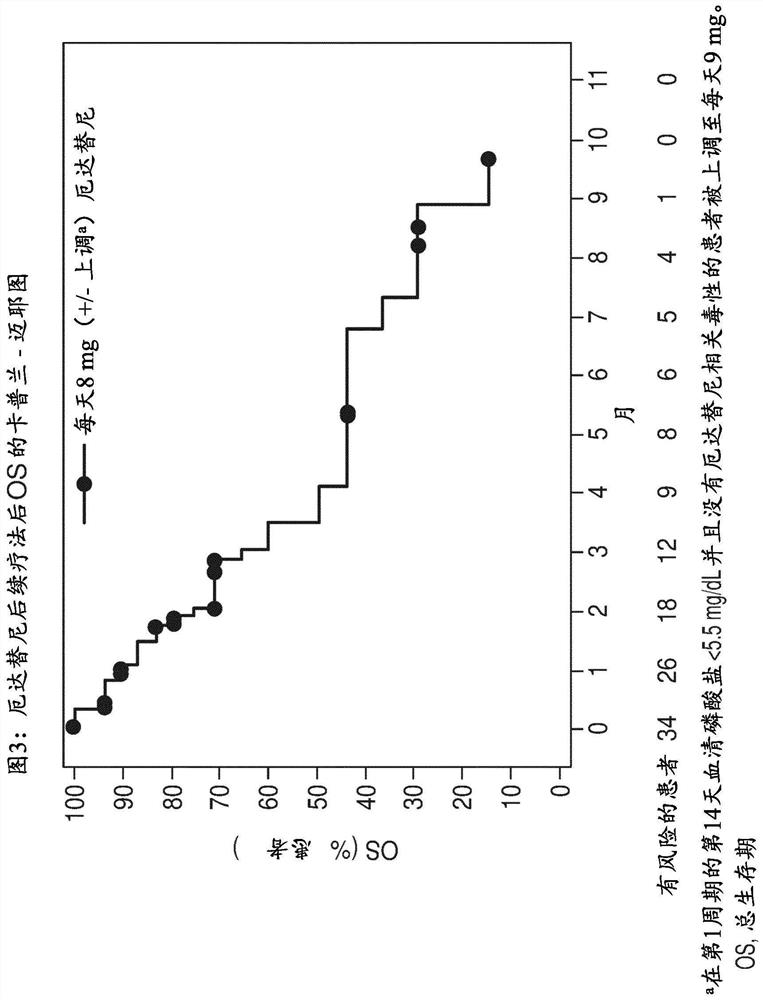
[0098] In this analysis, the clinical response to prior and subsequent therapy in FGFR-positive patients with mUC in the pivotal phase 2 study of erdafitinib was evaluated.
[0099] Research overview:
[0100] This is a retrospective analysis of data collected from patients randomized to regimen 3 (erdafitinib 8 mg once daily) of the phase 2 multicentre open-label study of erdafitinib (BLC2001; NCT02365597). This Phase 2 study is described, for example, in Loriot Y et al., N Engl J Med. 2019;25;381(4):338-348, which is incorporated herein by reference.
[0101] Patients with established FGFR2 / 3 mutation / fusion, locally advanced and unresectable or metastatic urothelial carcinoma that progressed during or after ≥ 1 line of prior chemotherapy or within 12 months of adjuvant / neoadjuvant chemotherapy , or ineligible for cisplatin use and chemotherapy-naïve.
[0102] Investigators report prior systemic therapy and subsequent lines of erdafit...
example 2
[0140] Example 2. Clinical Research
[0141] BLC2002 (NCT03473743) was to evaluate the safety of erdafitinib plus cilimumab (an anti-PD-1 monoclonal antibody) in subjects with metastatic or locally advanced urothelial carcinoma with selected FGFR gene alterations , efficacy, pharmacokinetics and pharmacodynamics phase 1b-2 study.
[0142] Phase 1b is the dose-escalation portion of the study, in which two dosing arms of erdafitinib (standard and alternative) were explored, while the intravenous (IV) dose of cilimumab was fixed. In the standard arm (DL1, DL2, or DL2A), erdafitinib and cilimumab were started simultaneously on day 1 of cycle 1 (C1D1). In the replacement group (DL1B or DL2B), the administration of erdafitinib was started on C1D1, but cilimumab was administered on day 1 (C2D1) of the second cycle (C2D1) after 1 cycle (4 weeks) (also known as erdatinib The 28-day trial run of Tini) started.
[0143] Blood for immune cell analysis was collected at four time points ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com