Combination therapy for treating cancer
A cancer and compound technology, applied in drug combinations, antibody medical components, chemical instruments and methods, etc., can solve problems such as drug resistance in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0066] Embodiment 1 A method of treating cancer comprising co-administering to a subject in need thereof:
[0067] a) a compound of formula (I) or a pharmaceutically acceptable salt thereof; and
[0068] b) allosteric inhibitors;
[0069] wherein formula (I) has the following structure:
[0070]
[0071] in
[0072] R 1 is hydrogen, C 1-4 Alkyl, C 3-6 Cycloalkyl, C 1-4 alkoxy or phenyl; and
[0073] R 2 is hydrogen, C 1-4 Alkyl, C 3-6 Cycloalkyl or halogen.
[0074] In one embodiment of the compound of formula (I), R 1 is hydrogen or C 1-4 alkyl.
[0075] In another embodiment of the compounds of formula (I), R 1 is hydrogen.
[0076] In another embodiment of the compounds of formula (I), R 2 is hydrogen or C 1-4 alkyl.
[0077] In another embodiment of the compounds of formula (I), R 2 is C 1-4 alkyl.
[0078] In another embodiment of the compounds of formula (I), R 2 is methyl or ethyl. In another embodiment, R 2 is methyl.
[0079] In another emb...
Embodiment approach 2
[0082] Embodiment 2 The method of Embodiment 1, wherein the compound of formula (I) is HQP-1351 or a pharmaceutically acceptable salt thereof, wherein HQP-1351 has the following structure:
[0083]
[0084]HQP-1351 is a novel, orally active, potent third-generation BCR-ABL inhibitor designed to efficiently target BCR-ABL mutants, including T315I, and it is being developed for the treatment of first and third-generation BCR-ABL Second-generation tyrosine kinase inhibitors (TKIs).
[0085] The chemical name for HQP-1351 is 3-(2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-(4-((4-methyl ylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)-benzamide.
[0086] The compound of formula (I) or the HQP-1351 compound or a pharmaceutically acceptable salt thereof can be prepared according to the production methods described in US Pat. No. 8,846,671B2, issued September 30, 2014, which is incorporated herein by reference, its entirety and for all purposes or methods similar ther...
Embodiment approach 3
[0087] Embodiment 3 The method of Embodiment 1 or 2 wherein the allosteric inhibitor is asciminib.
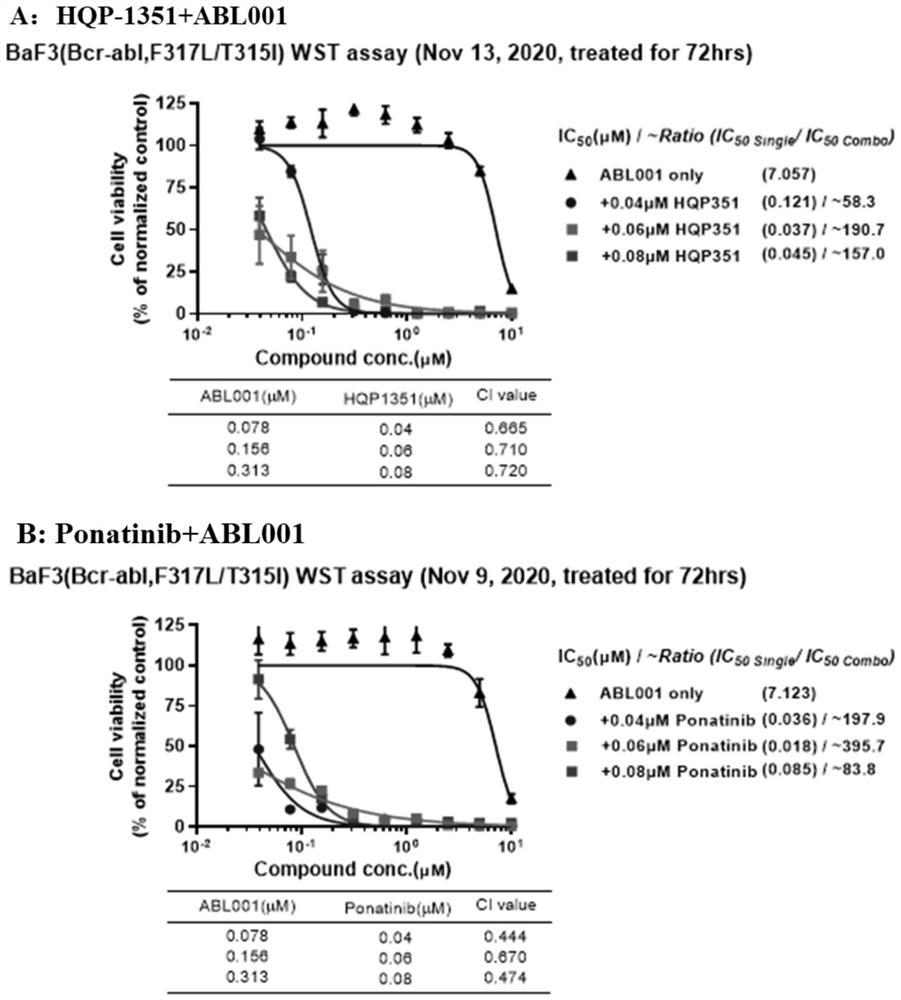
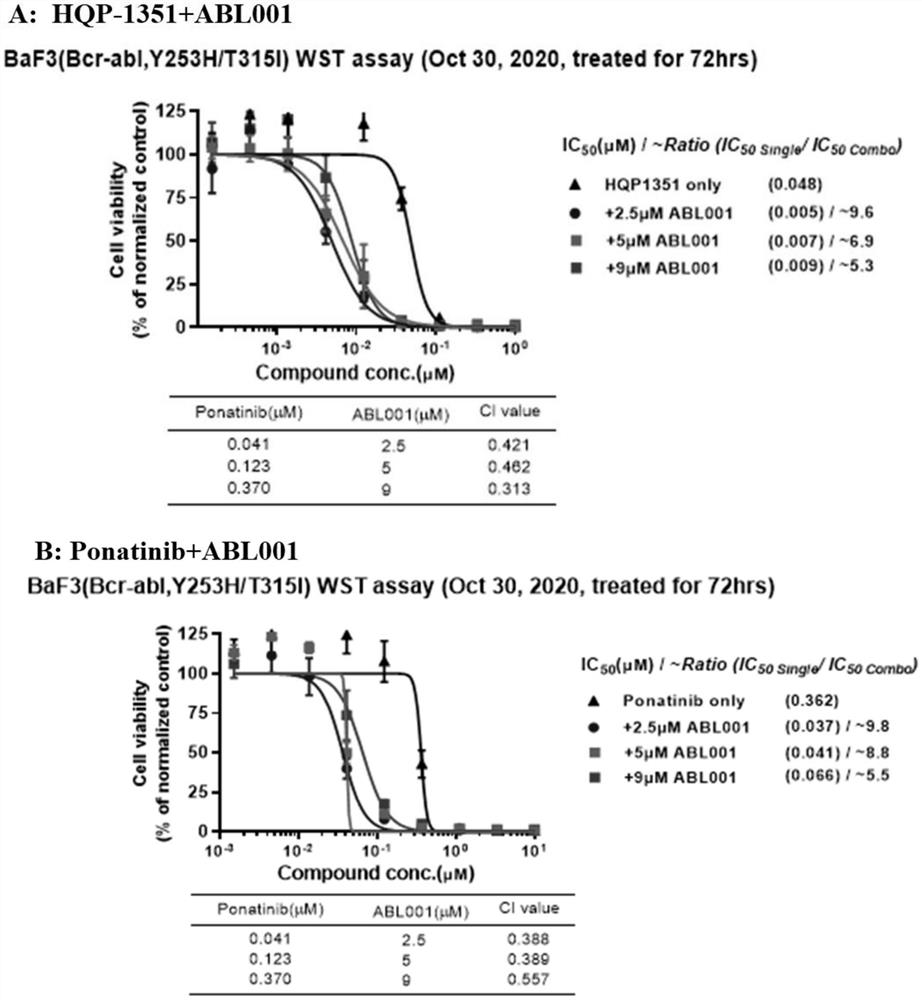
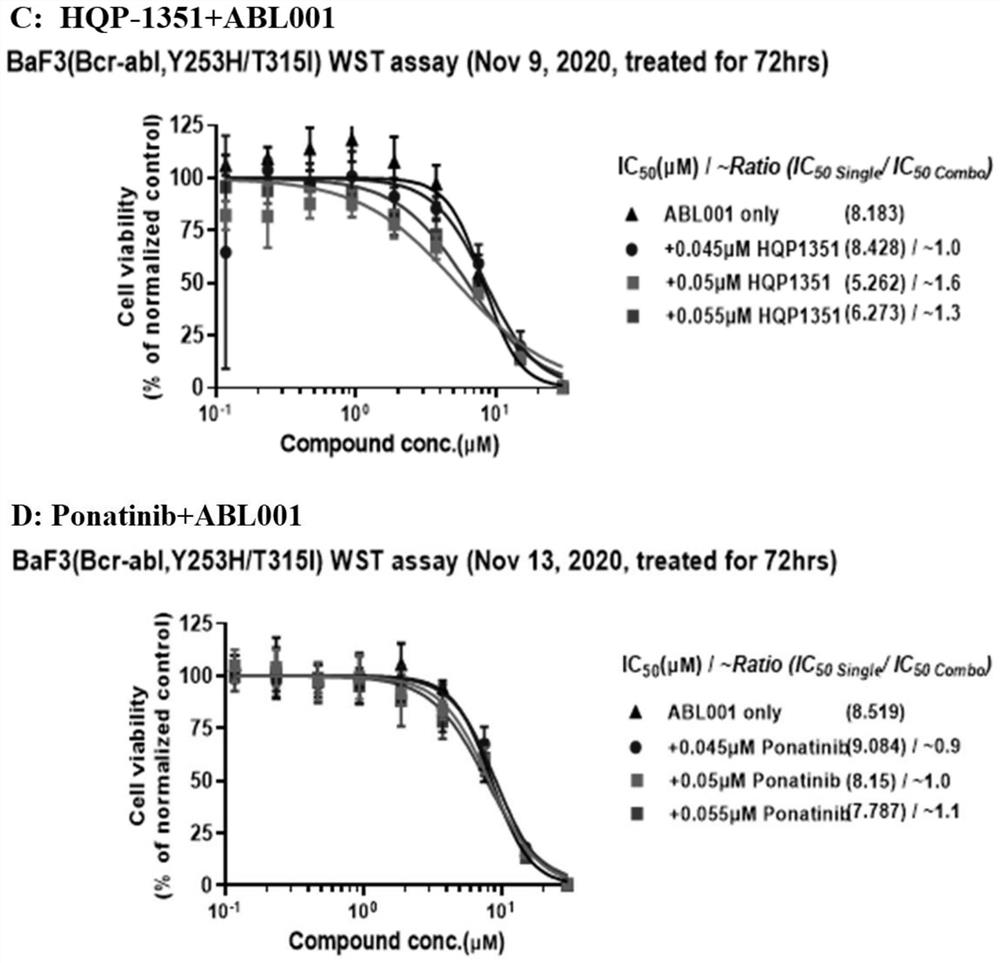
[0088] The present inventors found that HQP-1351, also known as orebatinib, enhanced the effect of allosteric inhibitors on the resistance conferred by the BCR-ABL compound mutation. Treatment with a tyrosine kinase inhibitor (TKI) targeting the BCR-ABL ATP binding site promotes recovery from Ph+ leukemia. However, the emergence of the checkpoint mutation T315I and compound mutants confers resistance to these TKIs. HQP-1351 is a new generation TKI targeting BCR-ABL. It is an ATP site inhibitor currently in development for r / rCML.
[0089] Asciminib is an allosteric inhibitor that targets the myristoyl binding pocket and downstream signaling of the BCR-ABL kinase. Studies have shown that combining asciminib with ponatinib can only overcome some of the resistance caused by the BCR-ABL compound mutant. The novel combination of HQP-1351 and asciminib, targeting the ATP pocket a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap