Maleimide carbazole compound and synthesis method thereof
A technology of maleimide and maleimide, applied in organic chemistry and other directions, can solve problems such as complex synthesis steps, and achieve the effects of good group positioning and selectivity, scientific process and wide source of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
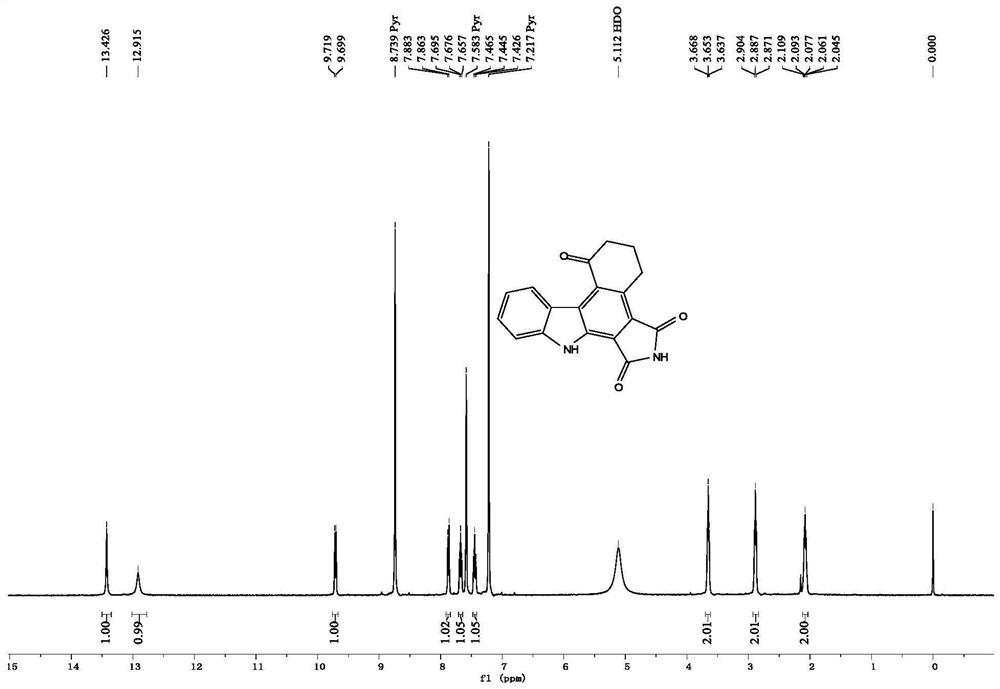
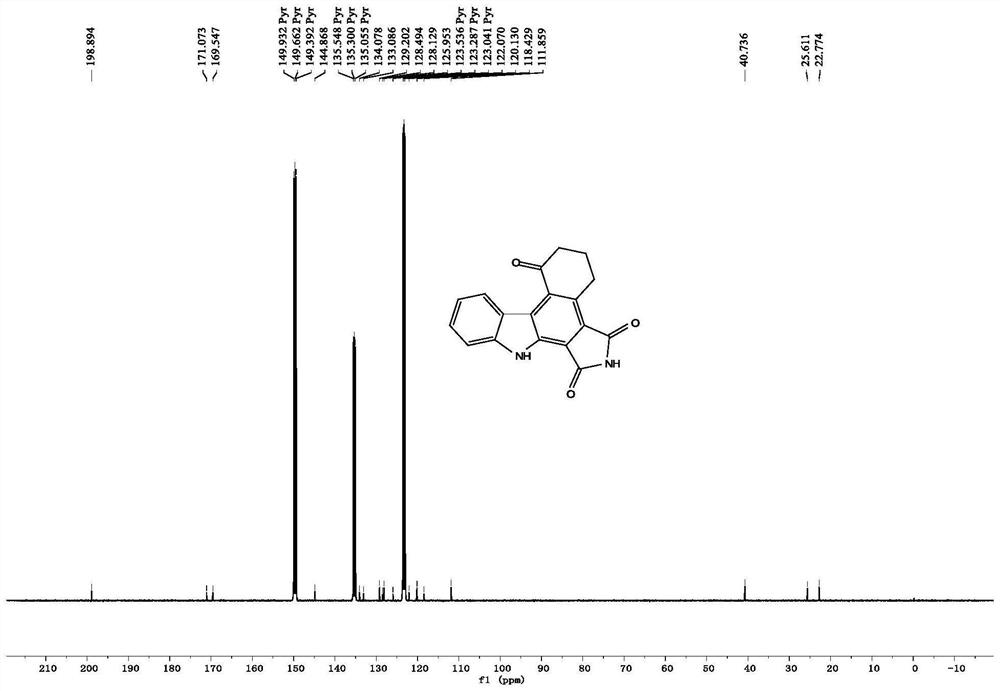
[0104] The nuclear magnetic data of the product of Example 1 are as follows:
[0105] 1 H NMR (400MHz, Pyridine-d 5 )δ13.43(s, 1H), 12.91(s, 1H), 9.71(d, J=8.0Hz, 1H), 7.87(d, J=8.0Hz, 1H), 7.68(t, J=7.6Hz, 1H), 7.45(t, J=7.8Hz, 1H), 3.65(t, J=6.2Hz, 2H), 2.89(t, J=6.6Hz, 2H), 2.11–2.05(m, 2H); 13 C NMR (100MHz, Pyridine-d 5 )δ198.9,171.1,169.5,144.9,134.1,133.1,129.2,128.5,128.1,126.0,123.5,122.1,120.1,118.4,111.9,40.7,25.6,22.8.
Embodiment 2
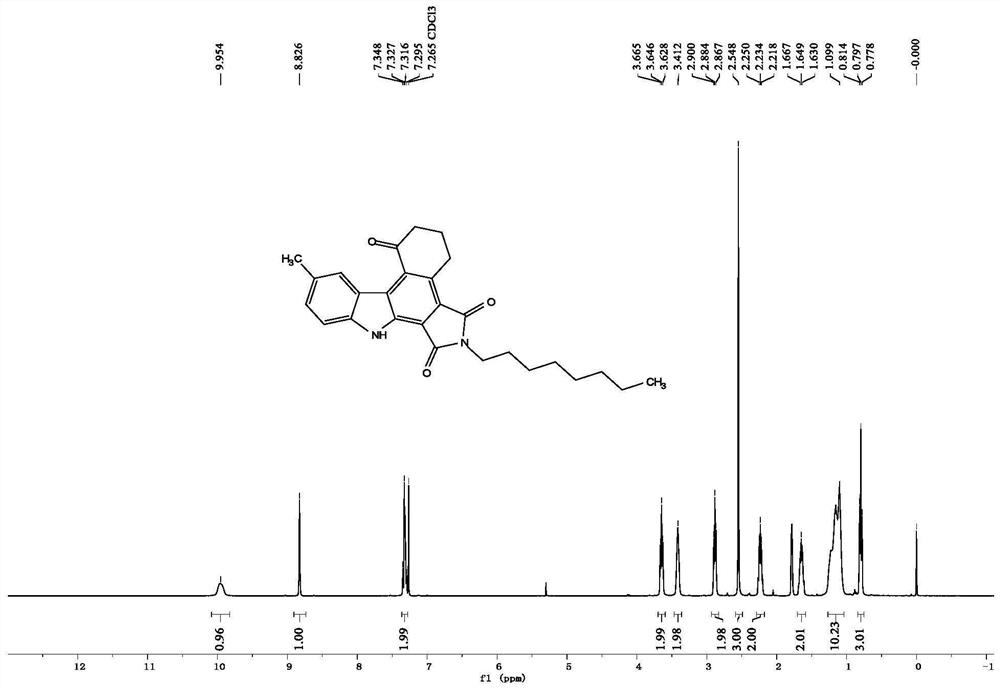
[0106] The nuclear magnetic data of the product of Example 2 are as follows:
[0107] 1 H NMR (400MHz, CDCl 3 )δ9.95(s,1H),8.83(s,1H),7.32(d,J=4.2Hz,2H),3.65(t,J=7.6Hz,2H),3.41(s,2H),2.88( t, J=6.6Hz, 2H), 2.55(s, 3H), 2.23(t, J=6.4Hz, 2H), 1.65(t, J=7.6Hz, 2H), 1.10(s, 10H), 0.80( t, J=7.2Hz, 3H); 13 C NMR (100MHz, CDCl 3)δ199.1,168.8,168.7,141.4,135.7,133.3,132.4,130.9,129.8,127.9,127.3,123.3,121.2,115.7,110.4,40.6,38.1,31.7,29.1,225.5,26.9 ,21.7,14.0.
Embodiment 3
[0108] The nuclear magnetic data of the product of Example 3 are as follows:
[0109] 1 H NMR (400MHz, CDCl 3 )δ9.50(s, 1H), 8.64(s, 1H), 7.36(d, J=8.8Hz, 1H), 7.21–7.18(m, 1H), 3.98(s, 3H), 3.68(t, J =7.4Hz,2H),3.50(t,J=6.2Hz,2H),2.92-2.84(m,2H),2.26-2.20(m,2H),1.67(d,J=7.4Hz,2H),1.35 –1.16(m,10H),0.84(t,J=6.8Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ199.3, 169.1, 168.6, 154.1, 138.1, 135.8, 133.8, 132.6, 128.3, 123.7, 121.8, 120.1, 116.2, 111.5, 109.1, 55.9, 40.7, 38.1, 31.8, 29.2, 2, 9.2, 28.8 , 22.5, 14.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com