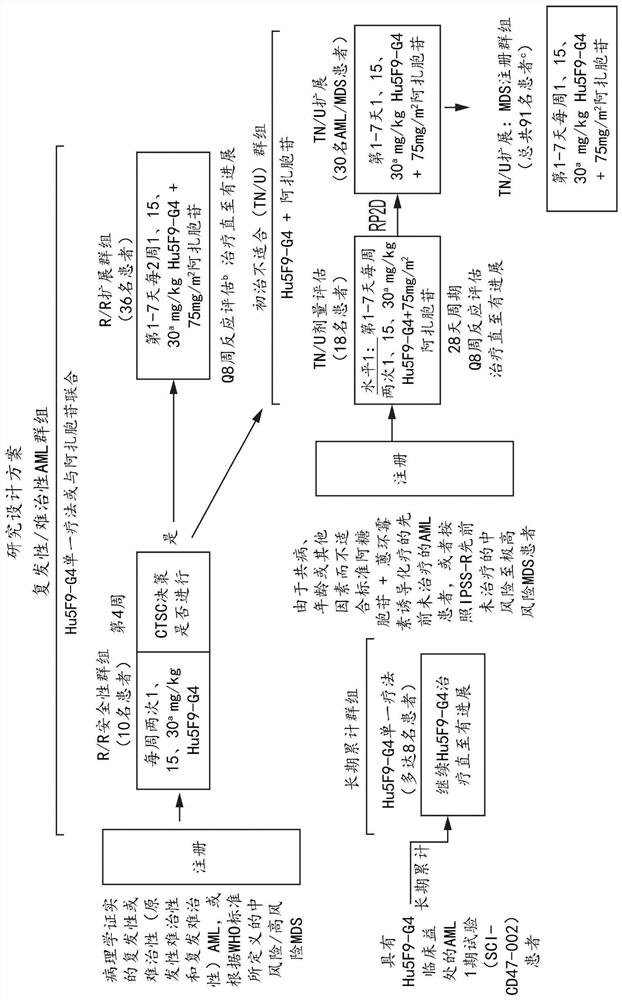
Combination therapy for treating myelodysplastic syndrome and acute myelogenous leukemia
A cancer treatment, cloning technology, applied in chemical instruments and methods, drug combinations, immunoglobulins, etc., can solve the problems of poor prognosis and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0650] Example 1: Hu5F9-G4 combined with azacitidine in patients with hematological malignancies
[0651] Introduction
[0652] Acute myeloid leukemia (AML) is a common hematological malignancy with an incidence rate that rises from 3:100,000 in young adults to more than 20:100,000 in older adults. Overall survival (OS) was 40% to 50% for patients younger than 60 years, but only 5% for patients older than 60 years. Most newly diagnosed AML patients are over the age of 60. In this patient population, standard induction chemotherapy is generally not an option due to increased treatment-related mortality due to age and comorbidities. The standard of care for AML patients who are ineligible for combination chemotherapy is treatment with a hypomethylating agent (azacitidine or decitabine) or low-dose cytarabine. Despite these first-line treatments, the median overall survival (OS) is only about 10 months. In all types of AML, disease recurrence is common and is the most com...
Embodiment 2
[0788] Example 2: Human Results
[0789] Safety of Hu5F9-G4 plus azacitidine
[0790] Forty-three patients were treated with Hu5F9-G4 plus azacitidine; 18 patients with MDS and 25 patients with AML. One patient (1 / 43: 2%) discontinued due to an adverse event (AE) of Hu5F9-G4 plus azacitidine (1DLT: G4 hemagglutination). The most common treatment-related adverse effects (TRAEs) were >15%: anemia (37%), neutropenia (26%), thrombocytopenia (26%). TRAE: Febrile neutropenia occurred in 1 patient (2%). No treatment-related infections were observed.
[0791] Efficacy of Hu5F9-G4 plus azacitidine
[0792] Shown in Table 5 are the MDS efficacy parameters and the number of responding patients in each parameter in the efficacy evaluable cohort and total treated patients.
[0793]
[0794] Shown in Table 6 are the AML efficacy parameters and the number of responding patients in each parameter in the efficacy evaluable cohort.
[0795]
[0796] Summarized in Table 7 is th...
Embodiment 3
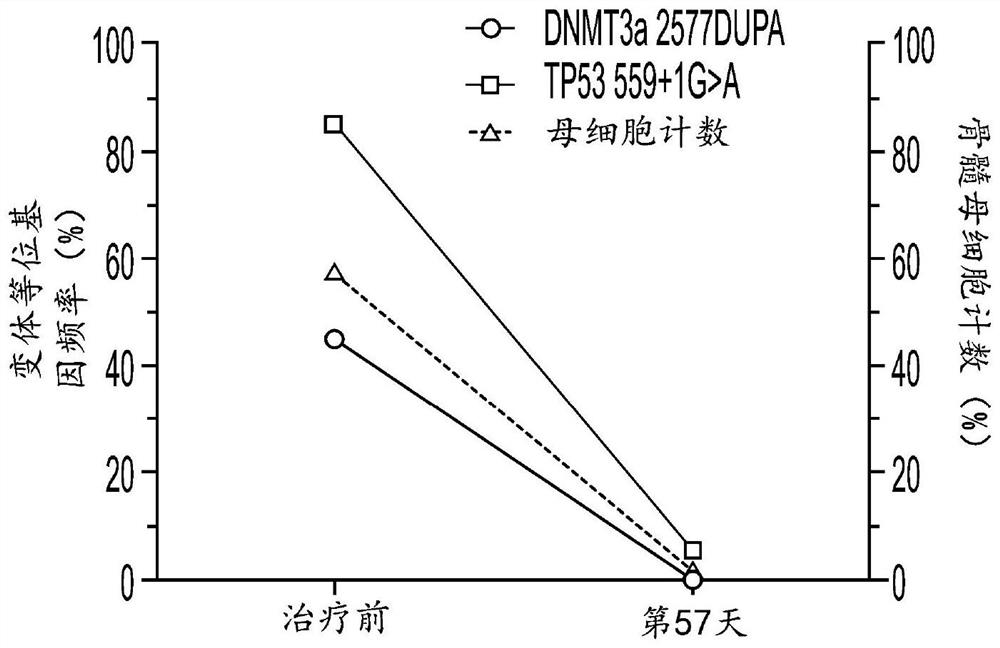
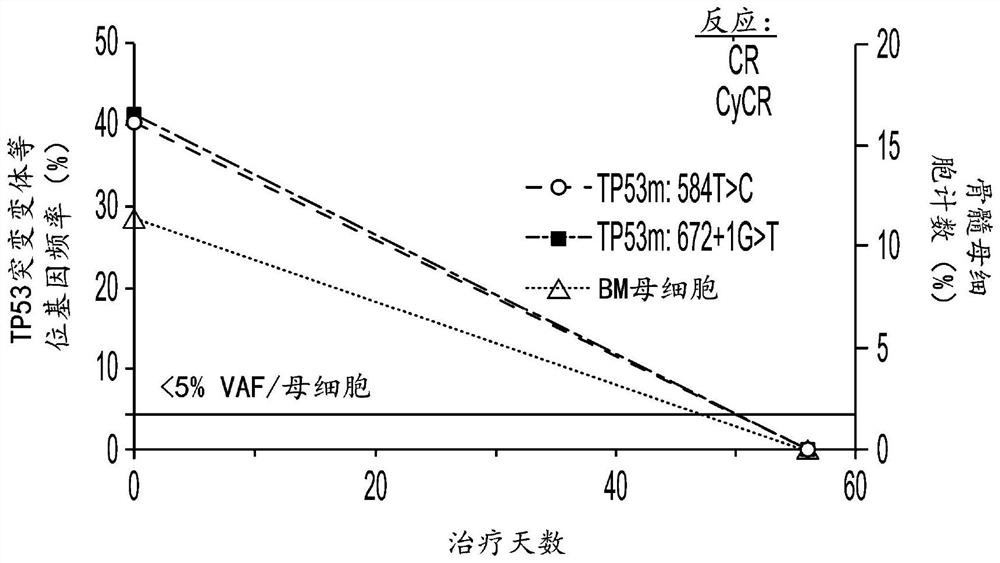
[0815] Example 3: Hu5F9-G4 and azacytidine reduce disease in AML patients with TP53 mutations
[0816] Clinical efficacy was shown in 9 untreated AML patients with TP53 mutations treated with Hu5F9-G4 and azacitidine. The overall response rate was 78%, of which 44% achieved CR and 33% achieved CRi. Additionally, deep responses were observed, as evidenced by a 67% CR rate by cytogenetics and 57% of patients achieving minimal residual disease negativity by flow cytometry. The median duration of response has not been reached, with a median follow-up of 6.9 months. Three additional AML patients with TP53 mutations treated with Hu5F9-G4 and azacitidine were identified. Clinical efficacy was also shown in these 12 treatment-naive AML patients with TP53 mutations. The overall response rate of n=12 TP53-mutant AML patients was 78%, of which 44% achieved CR and 33% achieved CRi.
[0817] Clinical efficacy was shown in 4 MDS patients with TP53 mutations treated with Hu5F9-G4 and aza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com