Triazene bridged azole-based crystal, preparation method thereof and application of triazene bridged azole-based crystal as energetic material
A kind of crystal and crystal technology, applied in the field of energetic materials, can solve the problems of environmental pollution by explosive products, insufficient detonation ability, complex synthesis process, etc., and achieve the effects of overcoming lead pollution, insufficient explosive performance, excellent stability and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
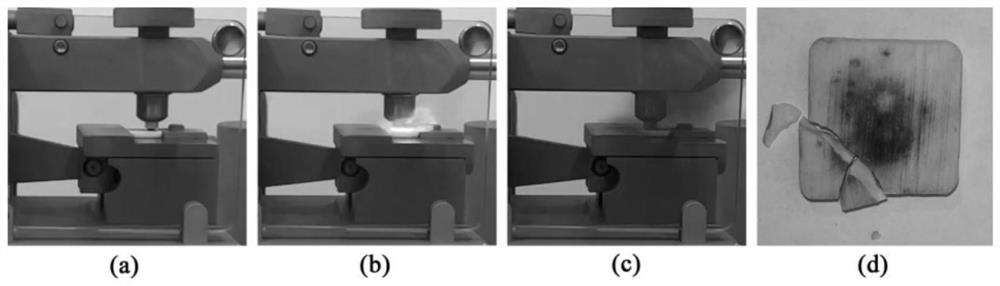
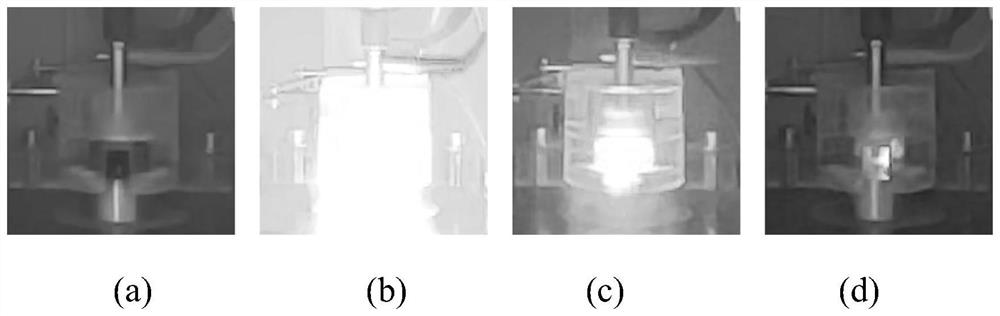
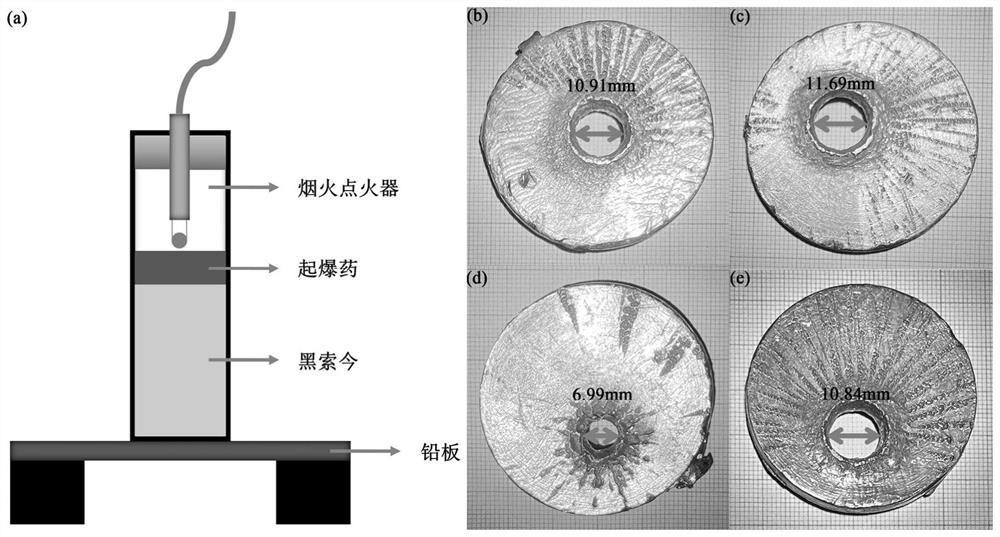
Image
Examples
Example Embodiment
[0042] Example 1 Compound Mn 3 C 8 H 12 N 34 Synthesis
[0043] Mn(ClO 4 ) 2 ·6H 2 O(72.39mg), NaN 3 (32.5 mg) and 1,3-bis(1-methyltetrazol-5-yl)triaza-1-ene monohydrate (136.31 mg) were added to 2 mL of deionized water to give a mixture which was heated At 90°C, the reaction was carried out for 36 hours, and then it was lowered to room temperature and filtered to obtain a large number of pink crystals, yield: 70%. Through X-ray single crystal diffraction test, the chemical formula of the pink crystal is Mn 3 C 8 H 12 N 34 .
Example Embodiment
[0044] Example 2 Compound Fe 3 C 8 H 12 N 34 Synthesis
[0045] Fe(ClO 4 ) 2 ·6H 2 O(217.70mg), NaN 3 (19.50 mg) and 1,3-bis(1-methyltetrazol-5-yl)triaza-1-ene monohydrate (113.6 mg) were added to 5 mL of deionized water to give a mixture which was heated to The reaction was carried out at 130° C. for 100 hours, then lowered to room temperature and filtered to obtain a large number of colorless crystals, yield: 75%. Through X-ray single crystal diffraction test, the chemical formula of the colorless crystal is Fe 3 C 8 H 12 N 34 of.
Example Embodiment
[0046] Example 3 Compound Co 3 C 8 H 12 N 34 Synthesis
[0047] Co(ClO 4 ) 2 ·6H 2 O(146.70mg), NaN 3 (26.00 mg) and 1,3-bis(1-methyltetrazol-5-yl)triaza-1-ene monohydrate (90.88 mg) were added to 6 mL of deionized water to give a mixture which was heated to The reaction was carried out at 120° C. for 36 hours, then lowered to room temperature and filtered to obtain a large number of red crystals, yield: 68%. Through X-ray single crystal diffraction test, the chemical formula of the red crystal is Co 3 C 8 H 12 N 34 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal stability | aaaaa | aaaaa |
| Impact sensitivity | aaaaa | aaaaa |
| Friction sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com