Binary co-amorphous substance and application thereof
An amorphous and binary system technology, applied in the field of medicine, can solve problems such as high energy consumption, high cost, and cumbersome preparation steps, and achieve high economic effect and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Spironolactone / L-Tryptophan Co-Amorphous
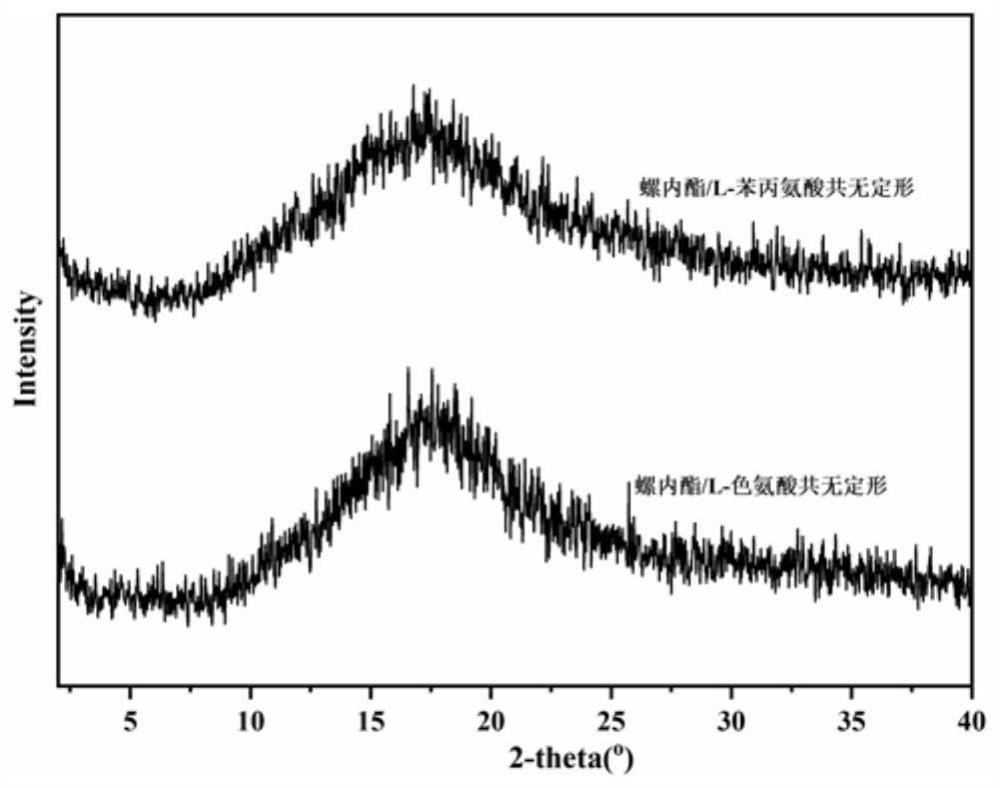
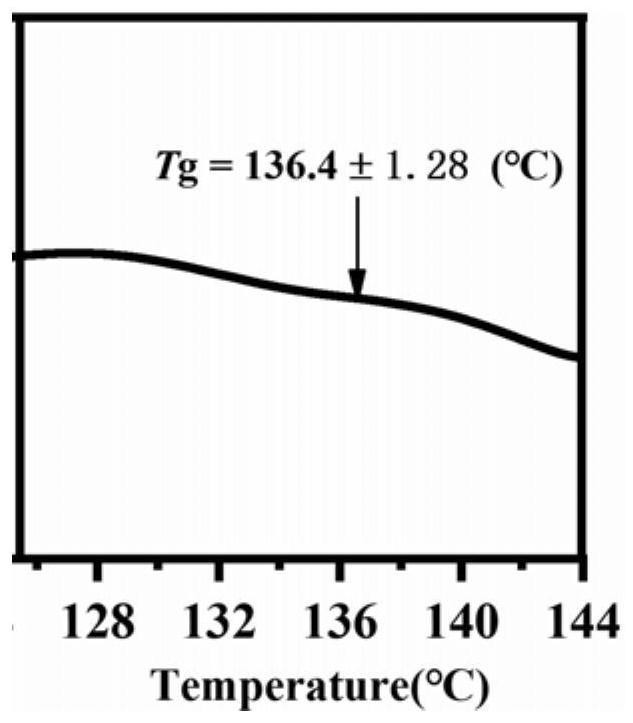
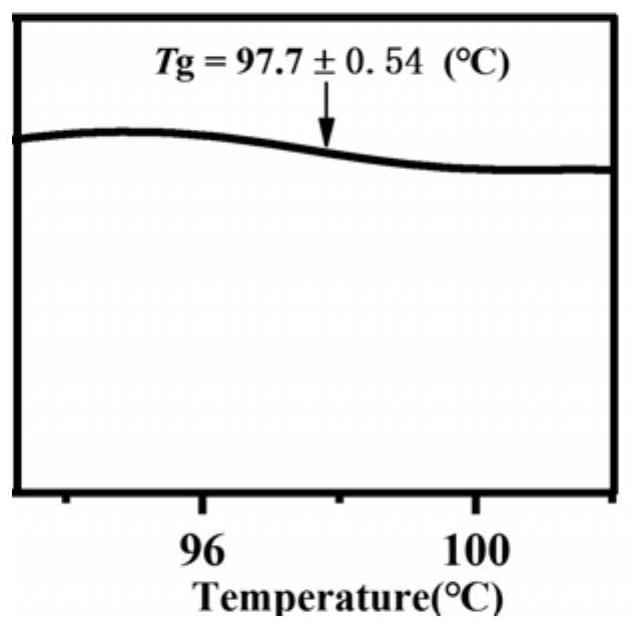
[0031] The spironolactone and L-tryptophan were placed in a 5mL ball milling jar according to the molar ratio of 1:1, and steel balls with a diameter of 5mm were added, the ball milling frequency was adjusted to 20Hz, and the ball milling time was 120min, and the solid spironolactone / L was directly obtained. - Tryptophan co-amorph. figure 1 Spironolactone / L-tryptophan co-amorphous PXRD powder diffraction pattern prepared in Example 1, by figure 1 It can be seen that the spironolactone / L-tryptophan co-amorphous X-ray powder diffraction pattern does not have the characteristic Bragg peaks of the crystal, and presents the characteristic steamed bread peaks of the amorphous. figure 2 For the spironolactone / L-tryptophan co-amorphous differential scanning calorimetry spectrum prepared in Example 1, by figure 2 It can be seen that the DSC curve of the spironolactone / L-tryptophan coamorph exhibits a single glass tran...
Embodiment 2
[0033] Preparation of Spironolactone / L-Tryptophan Co-Amorphous
[0034] The spironolactone and L-tryptophan were placed in a 5mL ball milling jar according to the molar ratio of 1:1, and a steel ball with a diameter of 8mm was added, the ball milling frequency was adjusted to 25Hz, and the ball milling time was 90min, and the solid spironolactone / L was directly obtained. - Tryptophan co-amorph. Figure 8 Spironolactone / L-tryptophan co-amorphous PXRD powder diffraction pattern prepared in Example 2, by Figure 8 It can be seen that the spironolactone / L-tryptophan co-amorphous X-ray powder diffraction pattern does not have the characteristic Bragg peaks of the crystal, and presents the characteristic steamed bread peaks of the amorphous. Figure 8 and figure 1 The comparison shows that the preparation of spironolactone / L-tryptophan co-amorph in Example 2 and the preparation of spironolactone / L-tryptophan co-amorph in Example 1 are the same substance. After testing, its differ...
Embodiment 3
[0036] Preparation of Spironolactone / L-Tryptophan Co-Amorphous
[0037] Put spironolactone and L-tryptophan in a 5mL ball milling jar according to the molar ratio of 1:1, add steel balls with a diameter of 10mm, adjust the ball milling frequency to 30Hz, and the ball milling time to 60min to directly obtain solid spironolactone / L. - Tryptophan co-amorph. Figure 9 The spironolactone / L-tryptophan co-amorphous PXRD powder diffraction pattern prepared in Example 3, by Figure 9 It can be seen that the spironolactone / L-tryptophan co-amorphous X-ray powder diffraction pattern does not have the characteristic Bragg peaks of the crystal, and presents the characteristic steamed bread peaks of the amorphous. Figure 9 and figure 1 The comparison shows that the preparation of spironolactone / L-tryptophan co-amorph in Example 3 and the preparation of spironolactone / L-tryptophan co-amorph in Example 1 are the same substance. After testing, its differential scanning calorimetry, infrared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com