Novel Hedgehog signaling pathway inhibitor
A solvate and compound technology, applied in the field of novel Hedgehog signaling pathway inhibitors, can solve the problems of unsuccessful treatment of medulloblastoma, muscle tremor, dysgeusia, etc., achieve good application prospects, prevent proliferation, and low clearance rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0070] Another aspect of the present disclosure relates to the preparation method of any of the compounds of formula (I) as described above, which specifically comprises the following steps:
[0071] a) the compound of formula III is obtained from formula II through condensation and cyclization reaction with o-phenylenediamine;
[0072] b) the compound of formula III is subjected to alkylation to obtain formula IV;
[0073] c) formula IV reacts with 1-Boc-piperazine to obtain the compound of formula V;
[0074] d) compound of formula V obtains the compound of formula (I) through reduction and addition reaction;
[0075] where X 1 , X 2 , R 1 , R 2 The definition is as described above; specifically, it can be carried out with reference to the following reaction formula:
[0076]
[0077] In a specific embodiment, more preferably, the present invention provides a compound of the following formula 0025A or a pharmaceutically acceptable salt thereof, or an isomer thereof,...
Example Embodiment
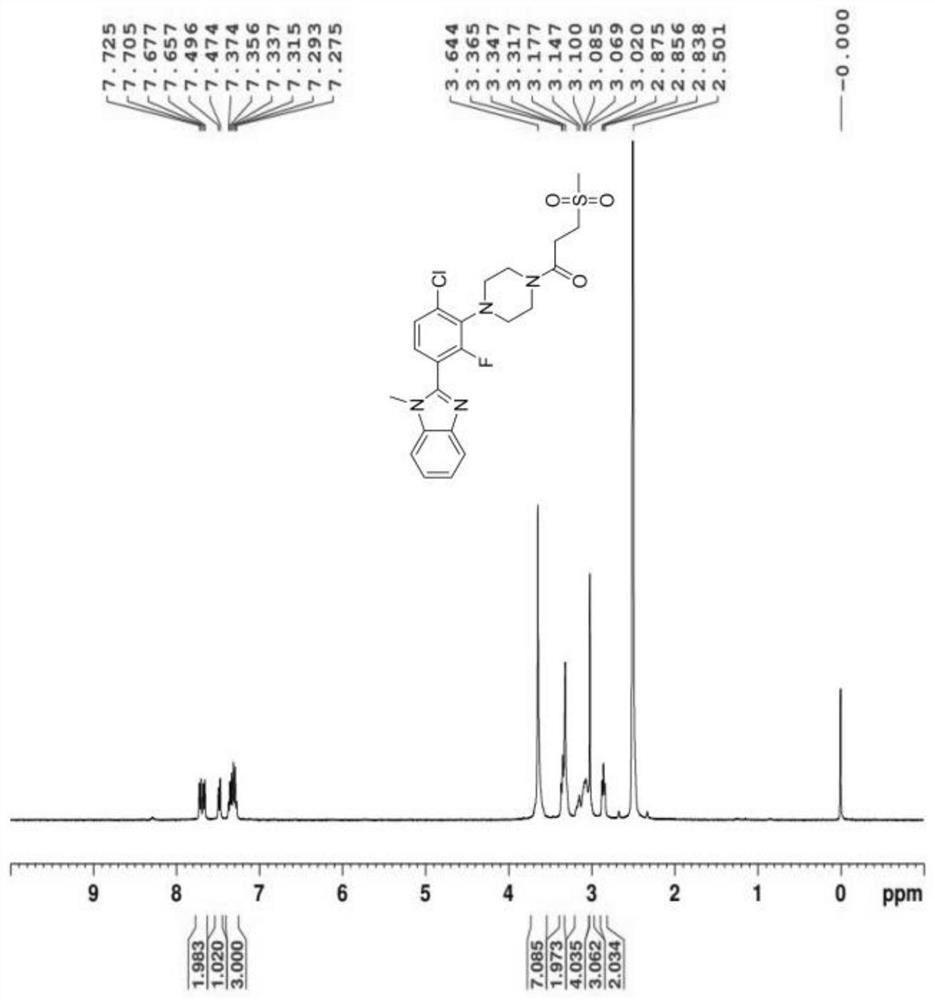
[0111] Example 1: Compound 1-(4-(6-Chloro-2-fluoro-3-(1-methyl-1H-benzo[d]imidazol-2yl]phenyl)piperazin-1-yl)- Synthesis of 3-(methylsulfonyl)propan-1-one (0025A)
[0112]
[0113] resolve resolution:
[0114] Step 1): Synthesis of N-(2-aminophenyl)-3-bromo-4-chloro-2 fluorobenzamide
[0115]
[0116]Compound 1 (6.5g, 25.9mmol), compound 2 (o-phenylenediamine, 5.6g, 51.8mmol) were dissolved in 50ml DMF, EDCI (5.9g, 31.07mmol,), HOBT (4.19g, 31.07mmol) were added DMAP (316 mg, 2.59 mmol), stirred at room temperature, after the reaction was complete, 150 ml of water was added, extracted with ethyl acetate three times (150 mL × 3), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and purified by silica gel column chromatography 5.2 g of the product compound 3 (N-(2-aminophenyl)-3-bromo-4-chloro-2fluorobenzamide) was obtained as a yellow solid with a yield of 58.8%.
[0117] Structural characterization of compound 1:
...
Example Embodiment
[0146] Example 2: In vitro pharmacokinetic detection of 0025A
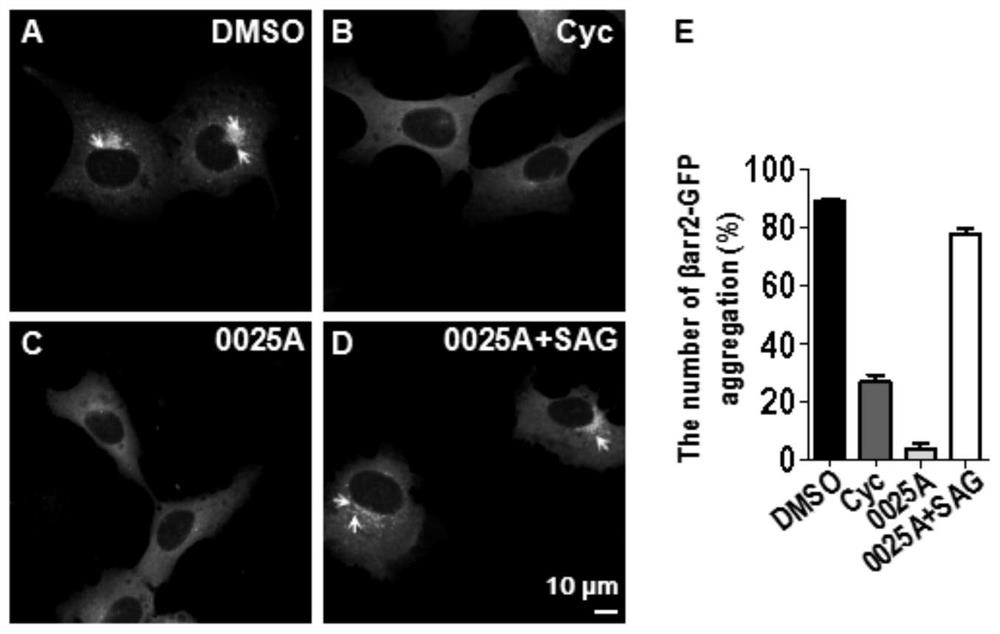
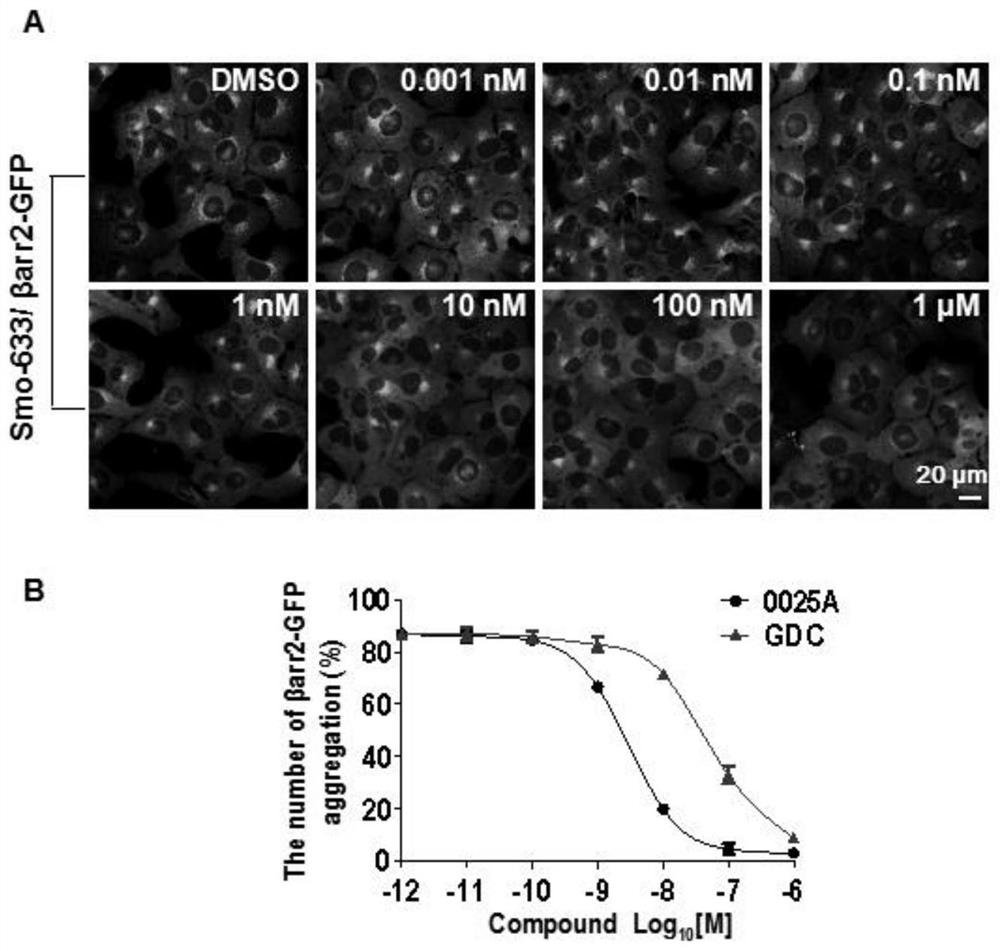
[0147] Using human liver microsomes, the metabolic stability of 0025A was tested in vitro, and compared with the positive control compounds (testosterone, propranolol) and negative control compounds (warfarin), 0025A showed low clearance and high metabolism Stability characteristics (Table 1).
[0148] Table 1 shows the in vitro pharmacokinetic analysis of 0025A:
[0149]
[0150] K, clearing coefficient; T 1 / 2 , half-life; Cl int , the intrinsic elimination rate; Cl app , the apparent half-life; Cl h , liver elimination rate; E h , the uptake rate.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap