CpGODN adjuvant and application thereof in antibody production
An adjuvant and antibody technology, applied in recombinant DNA technology, resistance to vector-borne diseases, DNA/RNA fragments, etc., can solve the problems that the target protein cannot be fully recognized and the cost of monoclonal antibodies is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Application test of CpG ODN adjuvant in antibody production
[0026] Step 1. Antigen preparation:
[0027] Human procalcitonin (PCT) recombinant protein recombinantly expressed in Escherichia coli was used as the immunogen, and the antigen concentration was 1 mg / mL; the basic immune antigen was 500ul recombinant PCT protein, mixed with 500ul Freund's complete adjuvant (Sigma, F5881), and carried out 1 minute phacoemulsification, repeated 3 times; 500ul recombinant PCT protein was used as the booster immunization antigen, mixed with 500ul incomplete Freund's adjuvant (Sigma, F5506), 1 minute phacoemulsification, repeated 3 times;
[0028] Step 2. Preparation of CpG ODN mixed adjuvant:
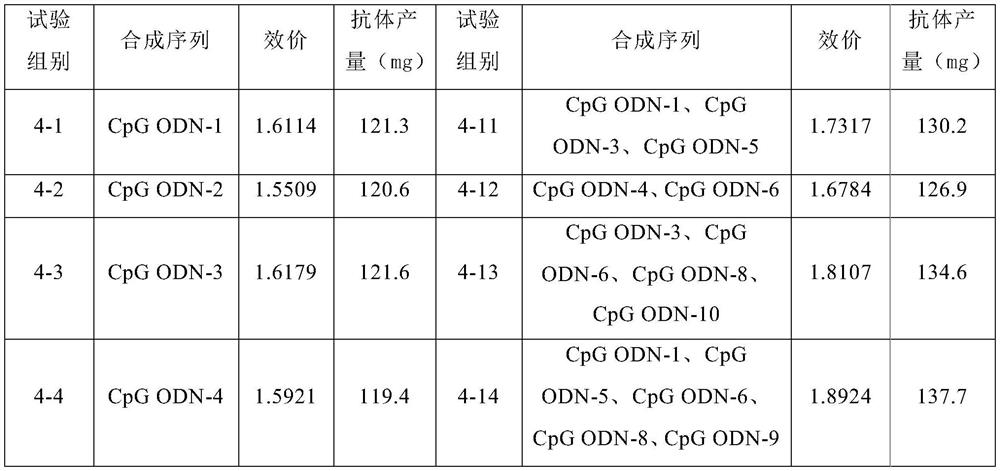
[0029] Synthesis of CpG ODN-1, CpG ODN-2, CpG ODN-3, CpG ODN-4, CpG ODN-5, CpG ODN-6, CpGODN-7, CpG ODN-8, CpG ODN-9, CpG ODN-10. Use ddH2O to dissolve into 5mg / mL mother solution, mix 0.1mL of each, and mix by vortex; before use, mix CpG ODN and Lipofectamine 2000 (Thermo, 11668019) in...
Embodiment 2
[0046] Application test of CpG ODN adjuvant in antibody production
[0047] Compared with Example 1, the immunization protocol in step 3 was replaced with the following protocol, and other steps were the same:
[0048] Optimized cycle group: booster immunization was performed every other week after basic immunization, and blood was collected one week after the fourth immunization. The basic immunization was inoculated with Freund's complete adjuvant and antigen emulsified, and CpG ODN mixed adjuvant was injected alone 3 days later. The booster immunization was inoculated with incomplete Freund's adjuvant and antigen emulsified, and CpG ODN mixed adjuvant was injected alone at the same time.
Embodiment 3
[0056] Result analysis:
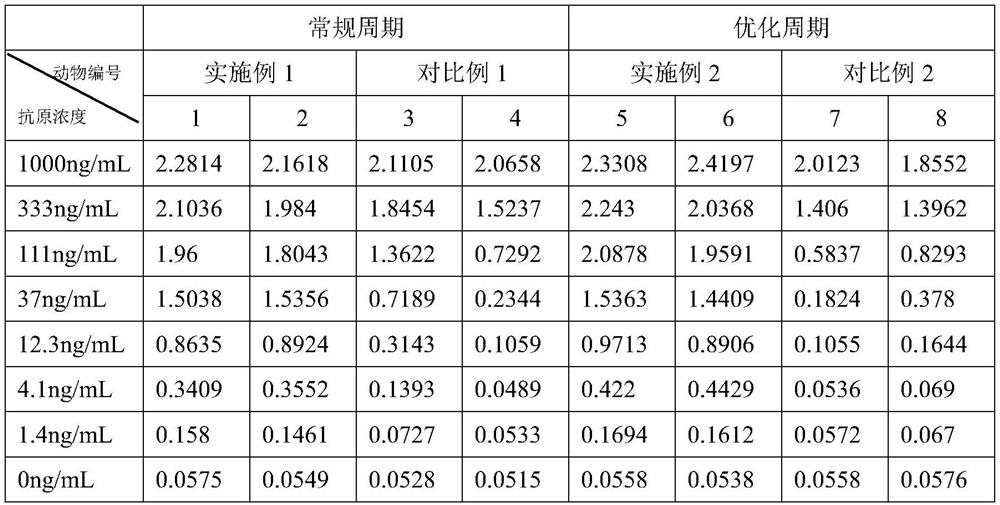
[0057] 1. Comparison of titers: Table 2 shows the titer data of the antibodies obtained in Example 1-2 and Comparative Example 1-2.
[0058] Table 2
[0059]
[0060] From the data in Table 2, it can be seen that the antibody titer results obtained in Example 1-2 are better than those obtained in Comparative Examples 1-2, and the antibody titer obtained in Example 2 is better than that obtained in Example 1. price result.
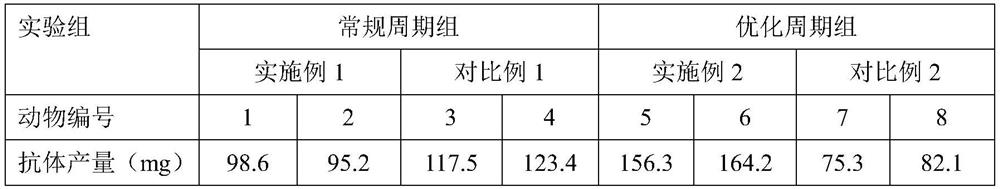
[0061] 2. Yield comparison: Table 3 shows the titer data of the antibodies obtained in Example 1-2 and Comparative Example 1-2.
[0062] table 3
[0063]
[0064] From the data in Table 3, it can be seen that the yield of antibody produced in Example 2 is higher than that of Example 1 and Comparative Examples 1-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com