Anti-CD45 antibodies and conjugates thereof
A technology of antibodies and antigens, applied in the direction of antibodies, anti-animal/human immunoglobulins, drug combinations, etc., can solve problems such as difficulty in ensuring HSC transplantation, hindering, and hindering implantation of exogenous HSC grafts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
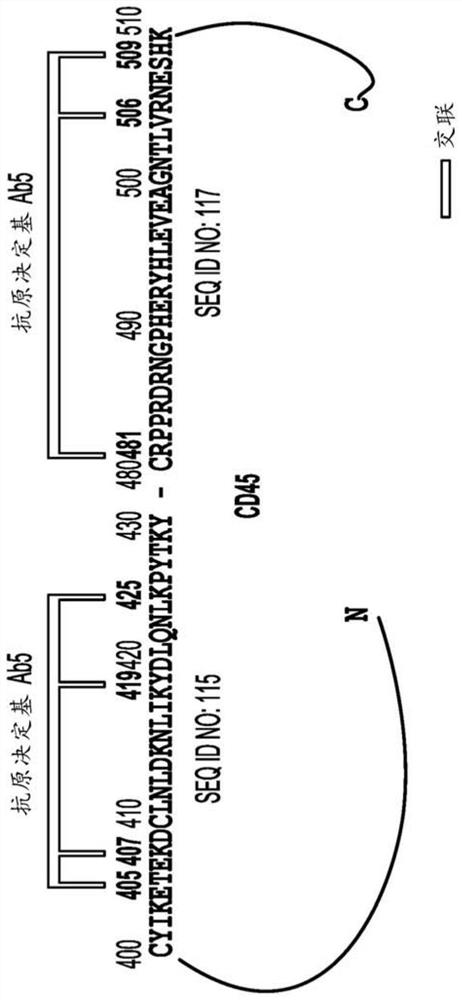
[1049] Example 1. Identification of anti-CD45 antibodies Abl-Ab7
[1050] A fully human library was screened and anti-human CD45 antibodies Ab1, Ab2, Ab3, Ab4, Ab5, Ab6 and Ab7 were identified. Each of the aforementioned antibodies is capable of internalization on CD45 expressing cells. The following examples provide additional details regarding library screening and the resulting antibodies.
[1051] Yeast display
[1052] Yeast display libraries displaying fully human antibodies (natural or synthetic) were screened for binding to the extracellular domain of human CD45 (isoform CD45RABC; Uniprot: P08575-3) and non-human primate (NHP) CD45. Yeast cells are selected that encode antibodies that bind to the recombinant CD45 antigen. Nucleic acid sequences representing antibodies from selected yeast cells are isolated according to techniques known in the art.
[1053] Specifically, screening was performed to identify human and NHP cross-reactive anti-CD45 antibodies. The firs...
example 2
[1059] Example 2. In vitro stability analysis of anti-CD45 antibodies
[1060] The stability of the antibodies identified in Example 1 was evaluated under various stress conditions. These studies identify VH / VL framework and CDR amino acids that can readily form post-translational modifications that can affect antibody heterogeneity and / or binding.
[1061]A two-week stability assay was performed by incubating Ab3 and Ab4 at 4°C, 25°C and 40°C for 15 days and then analyzing the antibodies by hydrophilic interaction chromatography (HIC). Briefly, 25 micrograms of the indicated antibodies were injected onto a Tosoh TSKgel Phenyl-5PW 7.5 mm ID x 7.5 cm 10 micron column (Cat. No. 07573) on a Waters ARCHPLC / UPLC system. The HIC elution profile of the antibody exhibits a relatively hydrophilic front peak with increasing thermal stress. This hydrophilic pre-peak indicates a potential post-translational modification (eg oxidation or deamidation) site. Peptide mapping was then perfo...
example 3
[1063] Example 3. In vitro binding assay of anti-CD45 antibodies
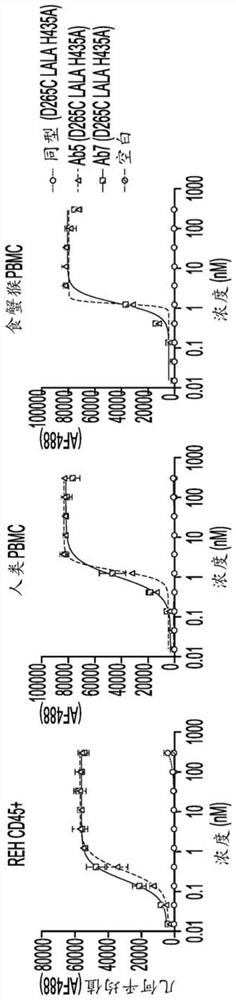
[1064] The antibodies described in Examples 1 and 2 were studied to determine their binding characteristics to human CD45 and to assess their ability to cross-react with rhesus and cynomolgus CD45.
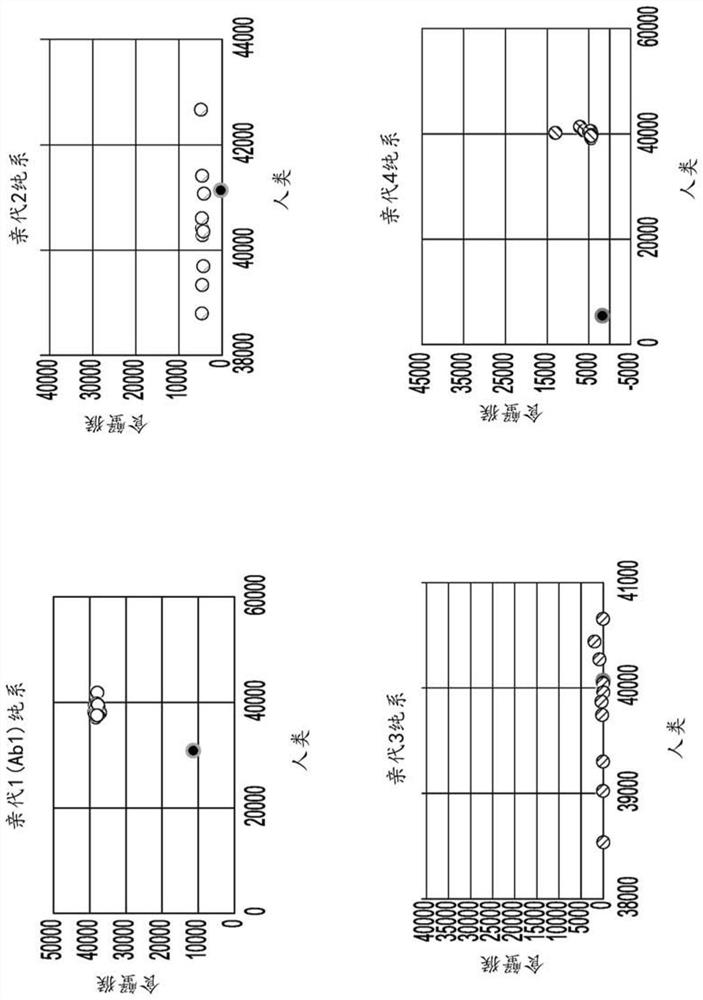
[1065] Antibody binding studies were performed using Biolayer Interferometry (BLI) using Pall ForteBio OctetRed96 in 1×PBS supplemented with 0.1% w / v bovine serum albumin at 25°C. Each purified human antibody was immobilized on an anti-human Fc biosensor (AHQ; Pall ForteBio 18-5001) and mixed with 50 nM of purified human, rhesus or cynomolgus CD45 extracellular domain (purified for affinity maturation). Lines Ab2-Ab7) were grown with 100 nM human CD45 and 300 nM rhesus or cynomolgus CD45 (for the parental clone Ab1).
[1066] Apparent monovalency was determined by partial complete fitting of each IgG to the purified human, cynomolgus or rhesus CD45 extracellular domain using a 1:1 binding model as calculated by Fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com