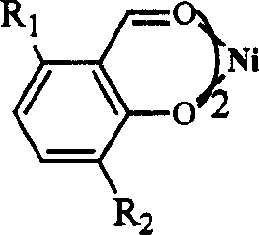
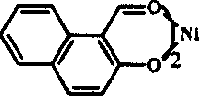
Catalytic system for preparing 6-C by di-propylene alkene
A catalytic system, six-carbon olefin technology, applied in the direction of hydrocarbons, hydrocarbons, physical/chemical process catalysts, etc., can solve the problems of complex systems, low activity, low activity, etc., and achieve cheap, easy-to-obtain and stable raw materials Good performance and wide application conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a 250mL autoclave, under propylene atmosphere, add 8μmol catalyst A, 20mL chlorobenzene, 10% Et 3 Al 2 Cl 3 Toluene solution (Al / Ni molar ratio is 300), stirred at 20°C for 10min, then adjusted the gauge pressure of propylene to 0.4MPa, and kept the pressure steady for 60min; the material was extruded, hydrolyzed, and the composition and content of the product were analyzed by GC. The results are shown in Table 1.
Embodiment 2
[0031] Example 2: 8 μmol of catalyst B, other experimental methods and conditions are the same as in Example 1. The results are shown in Table 1.
Embodiment 3
[0032] Example 3: 8 μmol of catalyst C, other experimental methods and conditions are the same as in Example 1. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com