New benzoyl urea compound and its synthesis and application
A technology for benzoyl urea and compounds, which is applied in the field of benzoyl urea compounds and their synthesis and application, and can solve problems such as high process requirements, increased overall synthesis difficulty, and uneconomical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
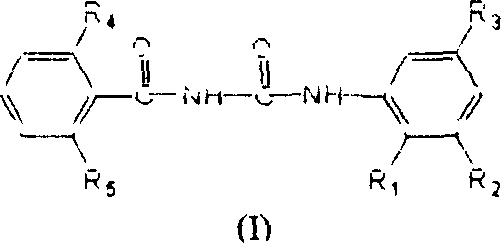
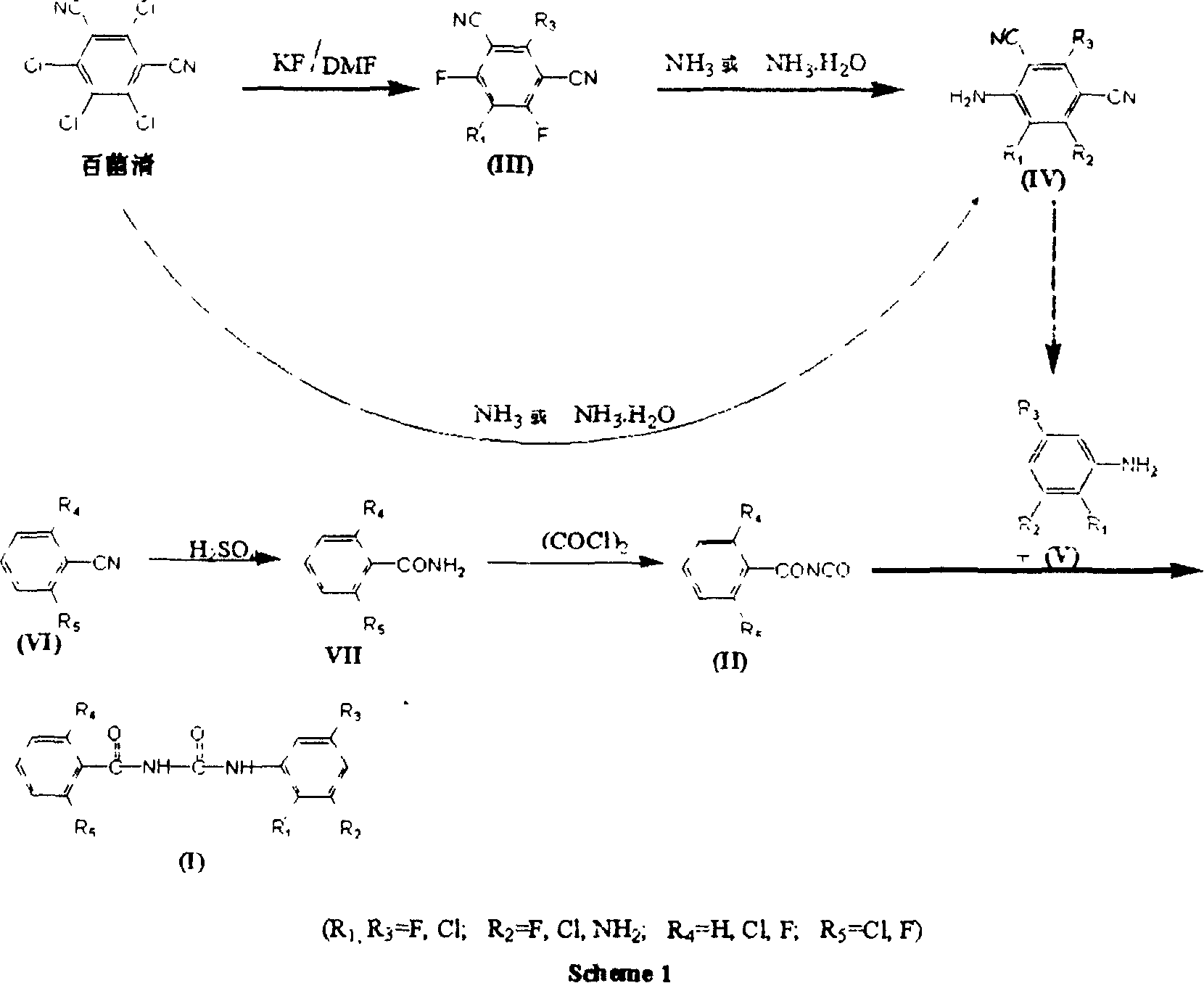
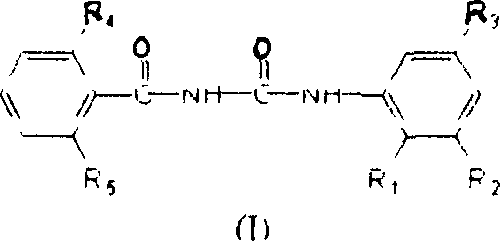
[0175] Preparation of N-(2,6-difluorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea: Weigh 0.1 mole of freshly prepared benzoyl isocyanate (II-2), Use dry 1,2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; weigh 0.1 mole of aniline derivative V-1, and also use dry 1,2-dichloroethane to make it Dissolve into a solution, then slowly drop it into the reaction bottle cooled with ice cubes while stirring, and immediately precipitate a white solid. After the heat release is complete, continue to react at room temperature for 3 to 4 hours, heat back to reflux water for 1 hour, cool, and filter with suction Get solid. Recrystallized with ethyl acetate to obtain 35.2 g of white needle-like crystals N-(2,6-difluorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea (I-1), producing The rate is 92.6%.
Embodiment 2
[0177] Preparation of N-(2-chlorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea: Weigh 0.1 mole of freshly prepared benzoyl isocyanate (II-1), dry it with 1 , 2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; take another 0.1 mole of aniline derivative V-1, and also use dry 1,2-dichloroethane to dissolve it into a solution , and then slowly dripped into the reaction flask cooled with ice cubes under stirring, and a white solid was precipitated immediately. After the exotherm was completed, the reaction was continued at room temperature for 3 to 4 hours, heated to reflux water for 1 hour, cooled, and suction filtered to obtain a solid. Recrystallized with ethyl acetate to obtain 34.7 g of white needle-like crystal N-(2-chlorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea (I-2), yield 91.8% .
Embodiment 3
[0179] Preparation of N-(2,6-dichlorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea: Weigh 0.1 mole of freshly prepared benzoyl isocyanate (II-3), Use dry 1,2-dichloroethane to dissolve and dilute it in a flask equipped with a stirrer; weigh 0.1 mole of aniline derivative V-1, and also use dry 1,2-dichloroethane to make it Dissolve into a solution, then slowly drop it into the reaction bottle cooled with ice cubes while stirring, and immediately precipitate a white solid. After the heat release is complete, continue to react at room temperature for 3 to 4 hours, heat back to reflux water for 1 hour, cool, and filter with suction Get solid. Recrystallization with ethyl acetate gave 37.4 grams of white needle-like crystals N-(2,6-dichlorobenzoyl)-N'-(2,3,5-trichlorophenyl)urea (I-3), producing The rate is 90.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com